Abstract
INTRODUCTION
Infantile haemangiomas (IH) are the most common vascular tumours in childhood. Over the past decade, treatment of IH has been revolutionised by the discovery of the effectiveness of beta-blockers in its treatment. We review our hospital’s experience with oral propranolol in the treatment of IH in an Asian population.
METHODS
We performed a retrospective review of the medical records and clinical photos of paediatric patients with IH treated with propranolol in a tertiary paediatric hospital in Singapore from January 2010 to February 2015.
RESULTS
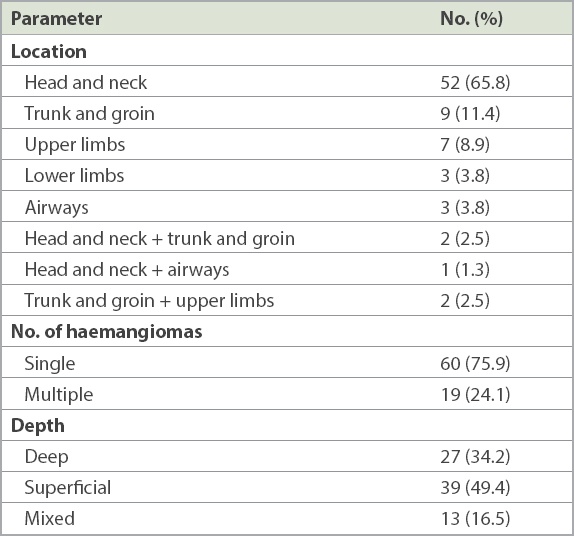
A total of 88 patients with IH treated with propranolol were identified over a five-year period, with 79 patients included in the final analysis. There was a predominance of female patients (75.9%) and preterm infants (41.8%) in our study population. The head and neck (65.8%), especially the orbital or preorbital region (45.6%), were the most common lesion sites in our cohort of patients. Mean age of onset was 2.3 ± 4.5 weeks of age, and mean age of starting propranolol treatment was 7.7 ± 10.5 weeks of age. 44.3% of patients experienced > 75% improvement, while 29.1% experienced improvement of 50%–75%. Response to treatment was influenced by the age of starting treatment.
CONCLUSION
Our study provides further evidence of the efficacy and safety of propranolol in the treatment of IH in an Asian population. Early treatment is recommended for optimal results.
INTRODUCTION
Infantile haemangiomas (IH) are the most common paediatric vascular tumours, occurring in 2%–4% of infants.(1-4) They are more common in Caucasian, female and premature infants.(5,6) Lesions are usually not present at birth and become evident during the first 3–6 weeks of life. This is followed by rapid growth during the first 4–5 months.(7) Although IH have the unique characteristic of self-involution after the first year of life, up to 10% of IH cases result in complications such as ulceration, bleeding, infection, airway obstruction and visual disturbance, warranting consideration for intervention.(8)
Historically, treatment options for complicated IH included intralesional and systemic corticosteroids, chemotherapeutic agents, laser therapy and surgical intervention.(9) These treatments potentially have considerable side effects. Propranolol is a non-selective beta blocker that is commonly used in patients with cardiac and thyroid conditions. In 2008, Léauté-Labrèze et al from the Bordeaux Children’s Hospital, France, reported their serendipitous observation of the rapid regression of IH in cardiac patients treated with propranolol.(10) Since then, propranolol has been the mainstay of treatment for complicated IH worldwide.
We reviewed the efficacy and safety of oral propranolol for the treatment of complicated IH in a tertiary paediatric hospital in Singapore with a mostly Asian population. Given that most published studies were largely conducted among a Caucasian population, we aimed to investigate for any biological factors that may affect the efficacy of treatment in our Asian population.
METHODS
We performed a retrospective descriptive study of paediatric patients with IH treated with propranolol in a tertiary paediatric hospital in Singapore over five years, from January 2010 to February 2015. The study was approved by the hospital’s ethics committee. All paediatric patients with IH who were treated with propranolol were identified from pharmacy records. Patients who were not treated with propranolol or received other treatment modalities such as laser therapy were not included in this study.
Patients were commenced on propranolol based on clinical indications, at the discretion of the attending dermatologist. Common indications included functional impairment, ulceration and large facial IH with potential cosmetic disfigurement. All babies below three months of age or with significant comorbidities were monitored in the paediatric inpatient ward for at least 24 hours upon commencement of propranolol. Regular monitoring of blood pressure and heart rate was performed during the admission for approximately 24 hours. Pre-feed blood sugar level was also recorded at least once during the admission
Data collection was performed via reviewing of case notes as well as serial digital photographs taken at each visit. The degree of improvement was assessed by the attending dermatologist based on clinical assessment, in comparison with earlier photographs. Parameters assessed were improvement in size, colour and elevation of IH.
All demographic and clinical characteristics of patients were summarised as mean ± standard deviation and frequency (percentage) for continuous data and categorical data, respectively. Efficacy of propranolol was judged based on improvement after treatment. Patients were categorised based on improvement status as > 75% improvement, 50%–75% improvement, 25%–50% improvement and < 25% improvement. These four groups were compared based on F-test and chi-square test for continuous data and categorical data, respectively. A p-value < 0.05 indicated statistical significance for all tests. SAS version 9.3 software (SAS Institute, Cary, NC, USA) was used for the analysis.
RESULTS
A total of 88 patients with IH who were treated with propranolol were identified from the database.
Table I
Characteristics of the patients (n = 79).
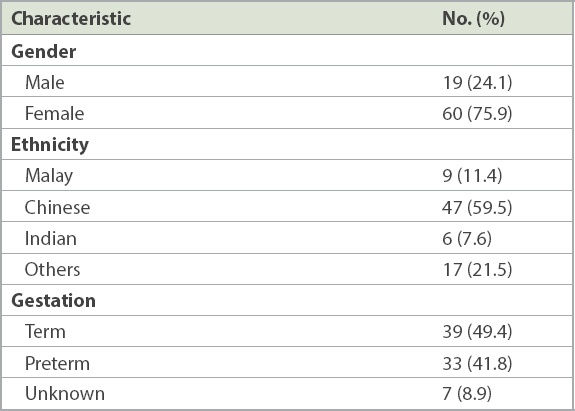
Table II
Clinical characteristics of the haemangiomas (n = 79).
With regard to response to treatment, 44.3% of patients experienced > 75% improvement, while 29.1% experienced improvement of 50%–75%. Only 26.6% of patients had < 50% improvement. Patients who experienced more significant improvement had been started on propranolol earlier in life. Patients treated before one year of age also responded better than those treated after one year of age (p = 0.002). For the 67 patients who started treatment before one year of age, 49.3% experienced > 75% improvement, 29.9% experienced 50%–75% improvement, 17.9% experienced 25%–50% improvement and only 2.2% had < 25% improvement. In contrast, in the 12 patients who started treatment after one year of age, up to 41.6% experienced < 25% improvement, 16.7% had 25%–50% improvement, 25.0% experienced 50%–75% improvement and only 16.7% experienced > 75% improvement. The response to propranolol was not influenced by gestational age (p = 0.7150), gender (p = 0.7367), age of onset (p = 0.7990), location (p = 0.1198), depth (p = 0.2328), size (p = 0.1952) or number of IH (p = 0.2642).
Of note, there were two patients with PELVIS (perineal IH, external genital malformations, lipomyelomeningocoele, vesicorenal abnormalities, imperforate anus and skin tag) syndrome and one patient with PHACES (posterior fossa brain malformations, segmental facial haemangiomas, arterial anomalies, cardiac abnormalities and coarctation of the aorta, eye abnormalities, and sternal cleft or supraumbilical raphe) syndrome. All three demonstrated good response, with > 75% improvement on treatment with propranolol. Additionally, 24.0% of patients had ulcerated IH and all improved with propranolol. For IH involving airways, response to treatment was assessed using radiology imaging.
Table III
Factors associated with degree of improvement following propranolol use.
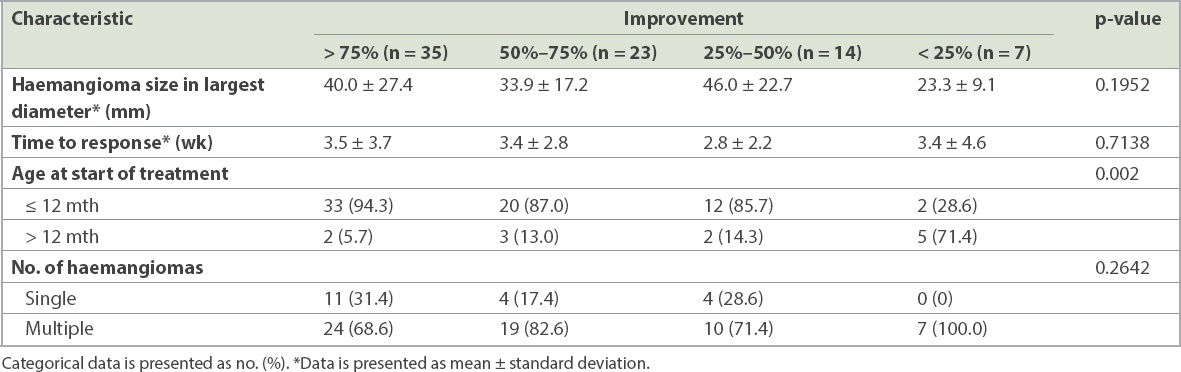
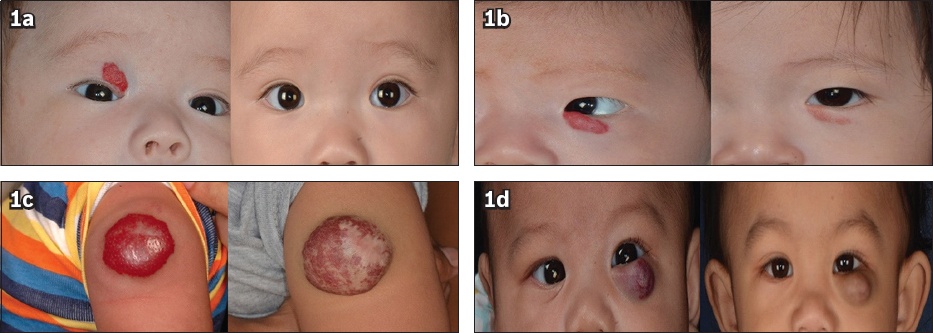
Fig. 1
Photographs show haemangiomas with (a) > 75%; (b) 50%–75%; (c) 25%–50%; and (d) < 25% of improvement after treatment with propranolol.
Data on side effects was collected via interviews with parents during each routine consultation by the attending dermatologist. There was a low incidence of side effects reported, which included sleep disruption in two patients, lethargy in one patient and bronchospasm in four patients. Two patients developed recurrent wheeze and required discontinuation of propranolol, after 12 months of treatment for one patient and after four months for the second. There were no reported episodes of hypotension, bradycardia or hypoglycaemia in our cohort.
DISCUSSION
Propranolol has traditionally been used in the paediatric population primarily for the treatment or prevention of cardiac arrhythmias, hypertension, outflow obstructions in congenital heart disease, and hypertrophic cardiomyopathy, at doses of as high as 8 mg/kg/day. Its antihypertensive effects result from decreased heart rate, decreased cardiac contractility, inhibition of renin release by the kidneys, and decreased sympathetic tone. It has been shown that in the pathogenesis of IH, elevated expression of pro-angiogenic factors, such as vascular endothelial growth factor, basic fibroblast growth factor and proliferating cell nuclear antigen, can stimulate endothelial growth and lead to dysregulated angiogenesis.(11) The overexpression of the glucose transporter 1 (GLUT-1) protein may also play a role in the pathogenesis of IH.(12) The mechanism of action of propranolol in the treatment of IH has yet to be clearly elucidated. Proposed hypotheses include vasoconstriction, decreased renin production, inhibition of angiogenesis, decreased plasmalemmal expression of GLUT-1, and stimulation of apoptosis.(13)
Similar to observations from other studies, our study found that early treatment with propranolol, especially in patients below one year of age, was associated with better response and degree of improvement.(14) However, a retrospective review of 18 patients by Vivas-Colmenares et al reported a favourable response beyond the proliferation phase of IH, with complete response in 72.2% of patients and partial response in 27.8%.(15) This suggests a further beneficial effect of propranolol on IH if it is commenced beyond the proliferative phase. Other than early initiation of treatment, no other factors have been found to be associated with greater efficacy of propranolol in our study population.
In a study by Sans et al, the mean duration of therapy was six months, with a range of 2–10 months.(16) Siegfried et al proposed that propranolol should be gradually tapered over a period of two weeks.(17) Chik et al proposed a treatment duration of 4–6 months, with a gradual 25% weekly decrease in propranolol dosage over four weeks.(18) In a meta-analysis conducted to analyse the safety profile and efficacy of propranolol among Chinese patients with IH, the mean age at initiation of propranolol was 4.7 months, with response noted within 24 hours on an average dose of 2 mg/kg/day.(19) Similarly, a more recent study by Léauté-Labrèze et al showed that propranolol at 3 mg/kg/day for six months was effective in the treatment of infantile haemangioma.(20) In our cohort, the mean duration of treatment was 11.0 ± 6.7 months. Unfortunately, data on recurrence was not available for our study. We also propose a treatment duration of at least six months up to about one year of age, based on our experience with the current cohort of patients. There is no consensus, however, on whether the drug should be decreased over a duration or stopped completely. At our centre, propranolol dosage is decreased over a duration of 1–2 months to observe for recurrence.
Known adverse events associated with propranolol, such as hypoglycaemia, hypotension, bradycardia and bronchospasm, occur infrequently.(20) Apart from bronchospasm occurring in four of our patients, none of them developed the more severe side effects of hypoglycaemia, hypotension or bradycardia, suggesting a very good safety profile at doses of less than 3 mg/kg/day. Milder side effects such as sleep disturbance as well as cool hands and feet do not require cessation of treatment.
Limitations of this study include the lack of a validated assessment for the evolution of infantile haemangiomas. Assessment of the degree of improvement was done by the attending dermatologist in comparison with previous photographic records, and thus there may be a risk of subjective bias. As this was a retrospective study, there could have been undocumented or missing data on treatment response as well as side effects. Furthermore, despite the five-year study period, the study population was relatively small.
In conclusion, our review reinforces results from previous studies that early treatment of complicated IH with propranolol is equally efficacious and safe in Asian populations. Given the favourable risk-benefit profile, propranolol should be the first-line therapy for infantile haemangiomas that require treatment.


