Abstract
INTRODUCTION
Acute fatty liver of pregnancy (AFLP) frequently causes liver failure in pregnant women. A better understanding of the clinical characteristics, management, outcomes and risk factors of AFLP is required, given its relatively high mortality rate. We aimed to describe the characteristics of AFLP, and further assess its outcomes and potential risk factors from the perspectives of the mother and fetus.
METHODS
This was a retrospective cohort study of 133 patients with AFLP hospitalised at four tertiary hospitals in China between January 2009 and April 2014.
RESULTS
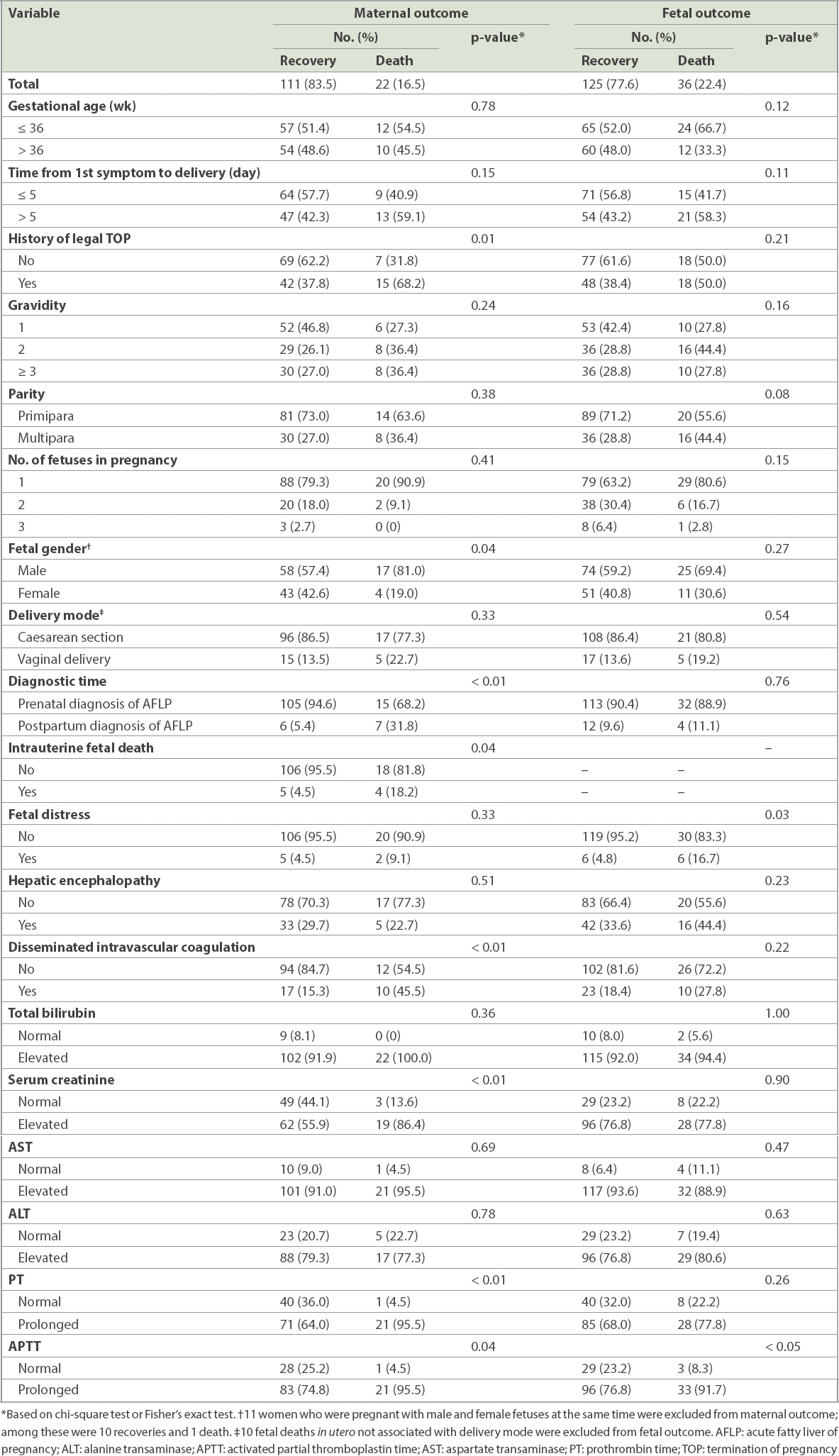
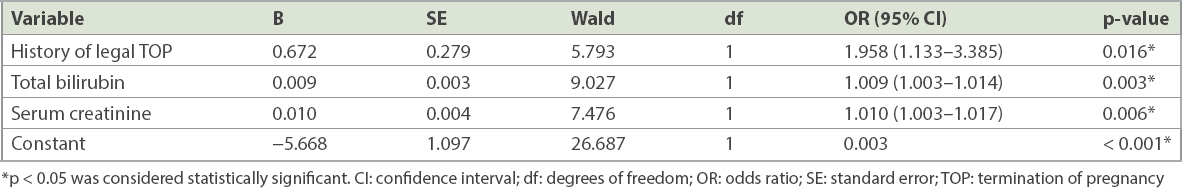
Among 133 patients, AFLP was diagnosed in the postpartum period for 13 (9.8%) patients. Potential factors influencing adverse maternal outcome were male fetus (p = 0.04), postpartum diagnosis of AFLP (p < 0.01), intrauterine fetal death (p = 0.04), disseminated intravascular coagulation (p < 0.01), prothrombin time (p < 0.01) and activated partial thromboplastin time (p = 0.04). The frequency of fetal distress (p = 0.03) and activated partial thromboplastin time (p < 0.05) were significantly higher in pregnancies with dead fetuses than in those where the fetuses survived. Independent risk factors for perinatal maternal mortality were history of legal termination of pregnancy (odds ratio [OR] 1.958, 95% confidence interval [CI] 1.133–3.385), total bilirubin (OR 1.009, 95% CI 1.003–1.014) and serum creatinine (OR 1.010, 95% CI 1.003–1.017).
CONCLUSION
Compared with total bilirubin and serum creatinine, history of legal termination of pregnancy appeared to be a greater risk factor for maternal mortality among patients with AFLP.
INTRODUCTION
Acute fatty liver of pregnancy (AFLP) is an uncommon, but potentially fatal, disease occurring in the late stage of pregnancy or in the early puerperium with microvesicular fatty infiltration of the liver, which can induce maternal multiorgan failure or even death of the mother and fetus.(1) As early as 1934, AFLP was depicted by Stander and Cadden(2) as ‘acute yellow atrophy of the liver’. Existing evidence shows that the aetiology of AFLP may involve oxidative stress on the liver, increased levels of serum arachidonic acid and abnormalities in the intramitochondrial processes of fatty acid oxidation, such as long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiencies caused by G1528C mutation.(1,3) The estimated incidence of AFLP ranges from approximately one in 7,000 deliveries to one in 20,000 pregnancies,(2-6) suggesting a low prevalence among pregnant women.
Although the incidence of AFLP is low, it remains a common cause of liver failure in pregnancy and may be accompanied by renal failure, coagulation disorders, hypoglycaemia, encephalopathy and, often, multiple organ dysfunction.(1,7) Both maternal and fetal mortality rates are significantly increased and vary between 1% and 20% despite optimal obstetric and medical management.(2,8) With acceptance of the importance of early recognition and diagnosis of AFLP as well as prompt delivery and intensive supportive care, maternal mortality has decreased from as high as 85%(9) to the current range of 12.5%–18%.(10) Simultaneously, neonatal mortality rate has also decreased and is in the range of 7%–66%.(3,11) Previous clinical studies on AFLP, largely based on a few patients due to its low prevalence, have found large variations in results vis-à-vis its epidemiology,(2-6) symptoms,(12) complications(12) and outcomes.(3,10,11,13) For instance, Cheng et al(14) found that, besides aetiology, other clinical characteristics of pregnancy (e.g. primiparity, male fetus and multiparous women) that had been identified as potential risk factors in some other studies(9,15) seemed to be partially statistically insignificant in their patients. Cheng et al also indicated that twin pregnancies might be a protective factor for maternal outcome.(14) Conversely, Knight et al(6) concluded that twin pregnancies were at higher risk of AFLP, similar to Davidson et al,(16) who demonstrated that triplet gestation could increase risk for AFLP.
In view of the insufficient understanding of AFLP and inconsistencies in the related literature, further studies involving larger patient populations are necessary to better guide clinical decision-making, improve prognosis, allow risk stratification and design clinical trials. Our study aimed to analyse a large sample of 133 patients with AFLP in order to: (a) summarise their clinical characteristics; and (b) further appraise the outcomes and potential risk factors of maternal and fetal mortality.
METHODS
We retrospectively analysed a consecutive series of 133 patients with AFLP hospitalised between January 2009 and April 2014 at the departments of critical care medicine or obstetrics at the following hospitals in China: Shandong Provincial Hospital Affiliated to Shandong University, Jinan; The First Affiliated Hospital of Nanchang University, Nanchang; Beijing Chao-Yang Hospital, Beijing; and Liaocheng People’s Hospital, Liaocheng. Patients’ data was collected from their medical records. The study was approved by the ethics committees at these hospitals.
The diagnosis of AFLP was determined based on both clinical features and laboratory findings, including: (a) symptoms of jaundice, anorexia, fatigue, nausea, vomiting and abnormal liver function during the third trimester of pregnancy; (b) characteristic laboratory findings (e.g. elevated alanine transaminase, bilirubin and serum creatinine levels), leucocytosis, prolonged prothrombin time, reduced fibrinogen and hypoglycaemia; (c) ultrasonography images or computed tomography (CT) examination showing fatty liver; and (d) liver biopsy sample, with characteristic pathological changes, where available. All patients exhibited six or more of the Swansea criteria (
Table I
Swansea criteria for the diagnosis of AFLP.
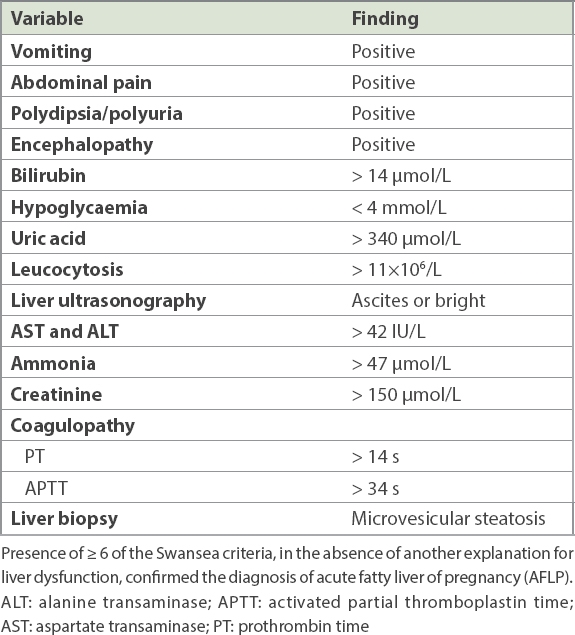
The diagnosis of acute liver failure based on clinical and laboratory criteria was made as follows: evidence of coagulopathy, international normalised ratio ≥ 1.5 or prothrombin activity ≤ 40%; serum total bilirubin ≥ 171 µmol/L or a daily rise ≥ 17.1 µmol/L; and any degree of mental alteration (encephalopathy) in a patient without pre-existing liver disease and illness < 2 weeks’ duration.(13) Acute renal failure (ARF) was defined as deterioration of renal function over days to weeks and serum creatinine ≥ 265.2 µmol/L, and acute kidney injury was defined as a deterioration in renal function and serum creatinine > 150 µmol/L.(13)
All statistical analyses were performed using IBM SPSS Statistics for Windows version 22.0 (IBM Corp, Armonk, NY, USA). Data was expressed as mean ± standard deviation, or frequency and percentages. Chi-square test or Fisher’s exact test was used for analysis. Logistic regression analysis was performed to analyse relative factors. A p-value < 0.05 was considered to be statistically significant.
RESULTS
In total, 140 patients were clinically diagnosed with AFLP during the study period. Among them, five patients were excluded as they did not meet the Swansea criteria and two patients were excluded because of obstetric cholestasis. Eventually, 133 patients with AFLP were enrolled in the study, among whom 13 (9.8%) were diagnosed in the postpartum period. Abdominal ultrasonography was performed for all patients and 57.1% had positive results. Biopsy was not done for any patients in view of their serious condition and lack of consent.
The mean maternal age of the patients was 27.1 years and mean gestational age was 36.1 weeks (
Table II
Demographic characteristics of patients (n = 133).
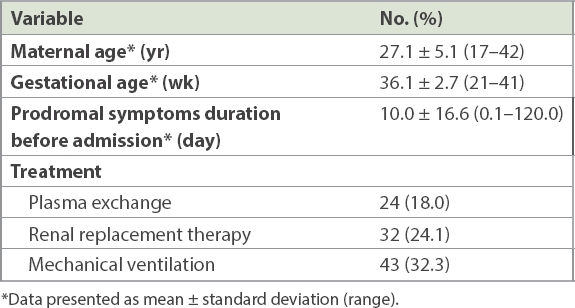
Table III
Clinical manifestations and complications of patients with acute fatty liver of pregnancy (n = 133).
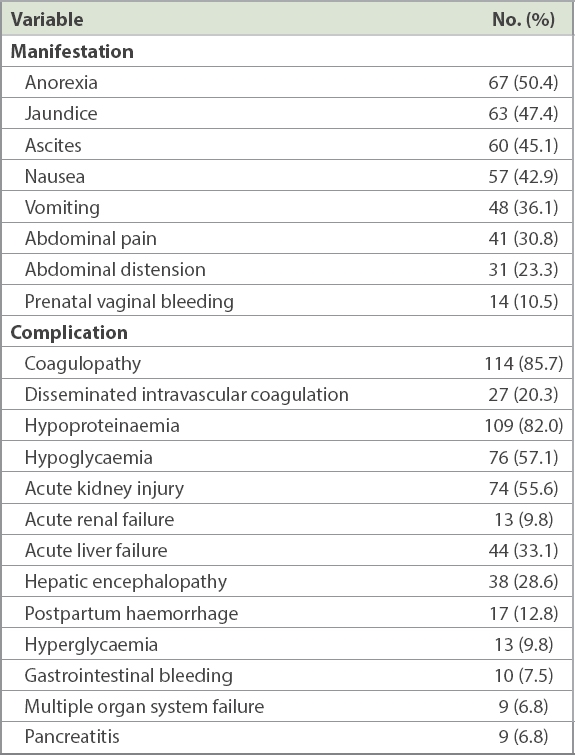
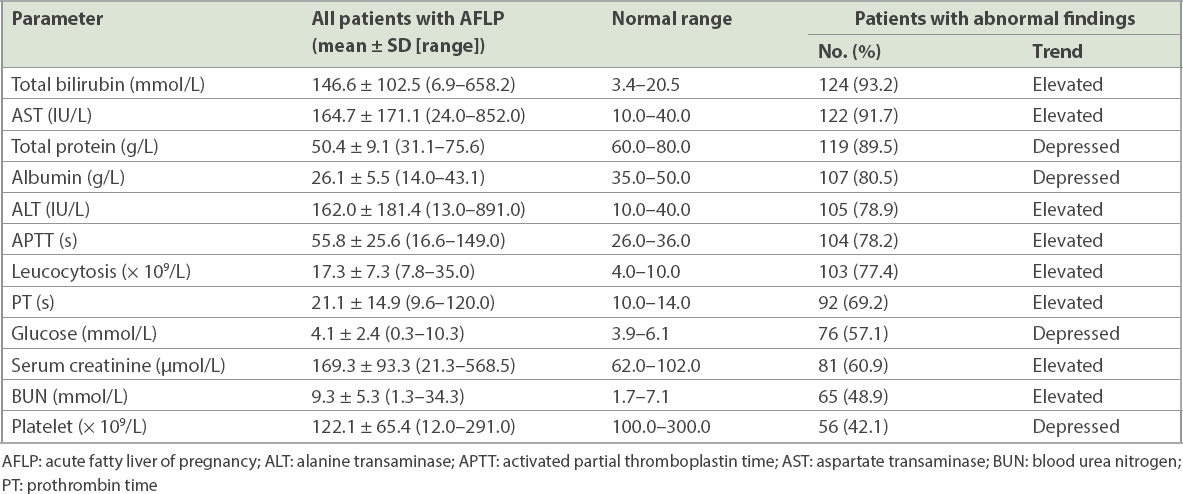
Table IV
Prenatal laboratory findings of patients with AFLP (n = 133).
As shown in
Table V
Stratification analysis of the influencing factors with maternal and fetal outcomes.
Multivariate logistic regression analysis was carried out for these variables, including gestational age, days from first symptom to delivery, frequency of TOP, gravidity and laboratory findings (
Table VI
Multivariate logistic regression analysis of factors influencing perinatal maternal mortality.
DISCUSSION
Symptoms of AFLP, especially in the early stages of disease, are atypical and can be overlooked. There is disagreement in the published literature regarding the symptoms and clinically common manifestations of AFLP, which included malaise, anorexia, nausea, vomiting, abdominal pain, jaundice, ascites, hypertension and abdominal distension.(4,12-14,17) We found that anorexia, nausea, vomiting and abdominal pain were the main prodromal symptoms, while progressive jaundice and ascites were common signs.
Although the gold standard for diagnosis of AFLP is liver biopsy, this was not done for any patient in our study due to the invasive nature of the procedure, the seriousness of the patients’ condition and lack of consent. Much like in our study, the diagnosis of AFLP has seldom been confirmed using liver biopsy in other reports.(6,11,13,14) Also, liver biopsy may not be necessary for the diagnosis of AFLP in most cases, especially for patients with typical clinical findings or severe coagulation disorders.(2,3)
Imaging evidence of fatty changes in the liver was commonly demonstrated by abdominal ultrasonography and CT. Knight et al found that classical features of ascites or bright liver were only seen in a quarter of patients who underwent abdominal ultrasonography.(6) All patients underwent ultrasonography in our study and 57.1% of patients had positive results. Similar to Knight et al’s findings,(6) few patients were diagnosed by CT in our study. Although CT may be considered a better option than ultrasonography when AFLP is suspected in a patient, ultrasonography is used more frequently for pregnant women, as it is difficult for these patients to accept CT given the possible harmful effects of radiation on the fetus. Wei et al(18) reported that the rates of positive AFLP diagnosis by ultrasonography and CT were 79.7% and 85.3%, respectively. However, the difference between the two methods was not statistically significant. In our study, we did not attempt to identify women with suspected AFLP using magnetic resonance imaging because of the undefined role of this modality in the diagnosis of AFLP.(19) However, the latest research has shown that magnetic resonance imaging-based liver fat quantification may be an effective tool to obtain a secure diagnosis of AFLP without liver biopsy.(20)
Early diagnosis, immediate delivery and comprehensive supportive treatment are the mainstay for the management of AFLP. The occurrence of complications is often associated with poor outcomes. Similar to earlier studies,(17) the major complications of AFLP observed among our patients were coagulopathy and hypoproteinaemia. Acute liver failure and ARF are the most significant and life-threatening complications of AFLP.(13) Among 133 patients, 74 (55.6%) patients experienced acute kidney injury, but only 13 (9.8%) patients developed ARF. Hence, the morbidity of ARF in our study was far less than in other reports.(12,17) Only 32 (43.2%) out of 74 patients with acute kidney injury in our cohort received renal replacement therapy. It is possible that early intervention, by way of renal replacement therapy, may have checked the further worsening of acute kidney injury in our patients, possibly resulting in the lower incidence of ARF. Ganesan and Maynard found that the cause of ARF in patients with AFLP may be related to early suppression of the b-oxidation of fats.(21)
In our series, 44 (33.1%) patients developed acute liver failure and 24 (18.0%) patients were treated with plasma exchange. Liver transplantation was not reported in our patients, although it has been suggested by Pereira et al(22) for patients with hepatic encephalopathy, severe metabolic acidosis or worsening coagulopathy. The role of liver transplantation in patients with AFLP is very limited due to the huge potential for recovery after delivery. Xiong et al have suggested that acute liver failure and ARF in patients with AFLP are reversible.(13) Related pathological findings were also reported by Rolfes and Ishak.(23)
Some clinical characteristics of pregnancy, such as primiparity, male fetus, multiple pregnancies and experiencing preeclampsia, were previously identified as potential risk factors for AFLP.(6,16,18,24) In our study, we identified history of TOP, male fetus, postpartum diagnosis of AFLP and intrauterine fetal death as the potential risk factors for adverse maternal outcome. Postpartum diagnosis of AFLP, which entails delayed comprehensive management of the condition, may be caused by the absence of early clinical manifestations and/or positive laboratory findings. While our data confirms the importance of early diagnosis of AFLP in pregnant woman, the diagnostic time appeared to have no effect on fetal outcome.
In our study, maternal and fetal mortality rates were 16.5% and 22.4%, respectively. This finding is similar to those reported previously(3,10) and highlights the vulnerability of patients with AFLP. There were 22 (16.5%) women with twin pregnancies in our study, which was similar to another study.(6) A retrospective study by Cheng et al(14) found significant differences between patients who survived and those who died with respect to twin pregnancies. The proportion of mothers with twin pregnancies in their study (28.1%) was much higher than in ours (16.5%) and another previous report (18.0%).(6) However, it is possible that their finding was incidental and, given the small size of their sample, cannot be generalised to all populations. Having twin pregnancies might be a potential protective factor for maternal outcome, but we did not find any statistical significance in our study.
The difference between fetal survival and death in this study was not statistically significant for women with multiparity, singleton pregnancies, male fetus, vaginal delivery, history of TOP and postpartum diagnosis of AFLP. However, the frequency of fetal distress was significantly higher among dead fetuses than those that survived, such that fetal distress might be a risk factor for fetal mortality (p = 0.03). Logistic regression analysis demonstrated that history of TOP, total bilirubin and serum creatinine were independent risk factors for maternal outcome. Importantly, the mortality rate among patients with a history of TOP was nearly 1.958 times that of patients without such history. Related research also shows that prothrombin time and international normalised ratio are risk factors for fatal complications in patients with AFLP, and perinatal mortality is associated with the level of fibrin degradation products.(25)
A key strength of our study was that data was sourced from tertiary hospitals and our sample was larger than that of previous reports. To the best of our knowledge, the potential risk factors we identified have not been previously described. Logistic regression analysis identified some independent risk factors of patient outcome, with a history of TOP being associated with worse and adverse maternal outcomes.
Nevertheless, our study was not without limitations. First, we did not evaluate the morbidity of AFLP, due to the deficiency of data on total pregnant women during the study period. Second, we could not explain why a history of TOP was associated with mortality for patients with AFLP. It is possible that this association between a history of TOP and poor maternal outcome in our cohort was a matter of chance and not causative. More extensive studies may be needed to establish or negate the existence of such a relationship. Third, given the retrospective nature of our study, we were unable to determine patient outcomes following discharge from the hospital.
To conclude, the main prodromal symptoms of AFLP were anorexia, nausea, vomiting and abdominal pain, while progressive jaundice and ascites were common signs among our patients. We found that male fetus, postpartum diagnosis of AFLP, intrauterine fetal death, disseminated intravascular coagulation, prolonged prothrombin time and activated partial thromboplastin time were potential risk factors for maternal outcome. Similarly, fetal distress and prolonged activated partial thromboplastin time were risk factors for fetal mortality. History of TOP, total bilirubin and serum creatinine were independent risk factors for maternal mortality.
ACKNOWLEDGEMENT
This study was funded by the National Natural Science Foundation of China (no. 81372473).


