Abstract
INTRODUCTION
Ovarian biomarkers have been shown to predict responses to controlled ovarian hyperstimulation (COH) during in vitro fertilisation (IVF) in predominantly Caucasian populations, with limited studies performed in Southeast Asian women in Singapore.
METHODS
We evaluated the performance of serum anti-Müllerian hormone (AMH), follicle-stimulating hormone and oestradiol levels, antral follicle count (AFC), body mass index, ovarian volume, and age to establish thresholds for the prediction of poor (< 4 oocytes retrieved) and excessive responses (> 19 oocytes retrieved) in 263 women undergoing COH. Univariate and multivariate logistic regression analysis and receiver operating characteristic curves were used to calculate probabilities for poor and excessive responders to COH.
RESULTS
36 (13.7%) and 50 (19.0%) women had poor and excessive response to COH, respectively. An AMH value of 0.69 ng/mL predicted poor ovarian response with positive likelihood ratio (LR) of 2.94, compared to an AFC of ≤ 5 when the positive LR is 2.36. Conversely, an AMH value of ≥ 3.06 ng/mL predicted excessive ovarian response with positive LR of 2.24, compared to an AFC cut-off of ≥ 12 with positive LR of 1.93.
CONCLUSION
AMH levels and AFC are equivalent in the prediction of both poor and excessive ovarian response in women undergoing IVF. Our study highlights the importance of establishing population-specific cut-off biomarker values so that protocols can be tailored to optimise IVF treatment.
INTRODUCTION
Ovarian reserve markers serve as an important tool in planning in vitro fertilisation (IVF) stimulation cycles in order to achieve an optimal number of oocytes for fertilisation. For example, a predicted high responder may be started on an antagonist cycle with a low follicle-stimulating hormone (FSH) dose and possibly an antagonist trigger for final oocyte maturation to reduce, albeit not eliminate, the chances of ovarian hyperstimulation syndrome (OHSS).(1,2) Conversely, for predicted poor responders or patients with diminished ovarian reserves, appropriate counselling on the possibility of cycle cancellation and an appropriate starting dose of FSH with adjuvants such as growth hormone may be considered.(3)
Common ovarian reserve markers used include age, early FSH and oestradiol (E2) levels, body mass index (BMI), ovarian volume, and antral follicle count (AFC).(3) Of these, AFC has the highest sensitivity in determining ovarian response.(4) More recently, a slew of studies have shown that serum anti-Müllerian hormone (AMH) has similar performance as AFC in identifying both poor and excessive responders in patients undergoing controlled ovarian hyperstimulation (COH) during IVF or intracytoplasmic sperm injection (ICSI) treatment.(5-7) Both AMH level and AFC also demonstrate a linear relationship with oocyte yields and have a strong correlation with each other.(8,9)
There are limited studies on ovarian reserve markers in response to controlled ovarian stimulation in multi-ethnic Southeast Asian women in Singapore.(10,11) This may be important, as it has been shown that AMH level may be influenced by ethnicity. For example, AMH levels can be 25% lower in Afro-American and Hispanic women compared to Caucasians, with genetic polymorphism being implicated.(12,13) In addition, FSH receptor polymorphisms affect different ethnicities, which may play a role in the prediction of ovarian response.(14) AMH measurements can also be affected by differences between generations of immunoassays; both technical aspects and a lack of standardisation between these assays may affect reliability and interpretation of AMH level results.(15-17)
Accordingly, we evaluated the performance of serum AMH, early follicular phase Day 2 or 3 FSH and E2 levels, AFC, BMI, ovarian volume and age as biomarkers for ovarian response to IVF treatment, and established thresholds for the prediction of poor and excessive responses to COH in patients undergoing IVF.
METHODS
Women undergoing fresh IVF/ICSI cycles at KK Women’s and Children’s Hospital in Singapore were prospectively recruited from March 2009 to January 2012. This study was approved by the SingHealth Centralised Institutional Review Board, Singapore. The patients’ past medical history and reproductive treatments were noted. All patients underwent a general physical and gynaecological examination, as well as ultrasonography of the pelvis. Human semen analysis was performed for the patients’ male partners prior to IVF/ICSI. We included women with primary or secondary subfertility diagnoses of: male factor, tubal factors, anovulatory cycles, endometriosis or unexplained fertility. Women who were more than 45 years of age or those with endocrine disorders such as diabetes mellitus and thyroid dysfunction were excluded.
After obtaining informed consent, all women underwent serum blood sampling to measure serum levels of AMH and FSH on Day 2 or 3 of the menstrual cycle. All samples were spun down immediately, stored in aliquots at −20°C and batch tested twice weekly on site at KK Women’s and Children’s Hospital using an in-house enzyme-linked immunosorbent assay (ELISA) method (AMH Beckman DSL kit, Webster, TX, USA), with inter- and intra-variability of 5.4% and 5.6%, respectively. Transvaginal two-dimensional ultrasonography was used (Aloka, Prosound α7; Hitachi Aloka Medical America, CT, USA) to determine AFC and ovarian volume prior to commencement of the IVF cycle. AFC was defined as the total number of follicles in both ovaries before the start of stimulation with an average diameter of two orthogonal planes measuring 2–9 mm on transvaginal ultrasonography, as described previously.(18) Serum AMH and AFC results were not used to determinine the starting dose of recombinant FSH used in the IVF/ICSI cycle.
All women underwent standard COH protocols employing either long agonist or short antagonist cycles with or without the use of combined oral contraceptive (COC) pills in a crossover protocol in anovulatory women, as determined by their own doctors. Women undergoing COC crossover commenced Microgynon 30 (ethinyloestradiol 30 mcg, levonorgestrel 150 mcg) on Days 1–2 of menses of the previous cycle for 14–16 days with a washout period of five days before crossing over to the agonist or antagonist cycle, as determined by their own doctors. Women undergoing the long gonadotropin-releasing hormone (GnRH) agonist protocol were treated with GnRH agonist (0.5 mg leuprorelin acetate; AbbVie, North Chicago, IL, USA) for 14 days, starting from menstrual cycle Day 21 or after completion of 21 days of COC. Serum E2 level < 50 pmol/mL on cycle Day 2 and endometrial thickness < 4 mm indicated adequate pituitary supression before COH was initiated with recombinant FSH (follitropin beta, Puregon; Schering-Plough, Kenilworth, NJ, USA) at different age-specific doses (< 35 years: 200 U per day, 35–38 years: 250 U per day and > 38 years: 300 U daily), with a lower dose of 150 U daily for women diagnosed with polycystic ovarian syndrome. Women undergoing the GnRH antagonist protocol commenced COH with recombinant FSH on menstrual cycle Days 2–4, with the addition of daily GnRH antagonist (0.25 mg ganirelix acetate; Merck Sharp & Dohme, Kenilworth, NJ, USA) from Day 5 or 6 of stimulation until the day of human chorionic gonadotropin (hCG) trigger. hCG trigger (intramuscular 10,000 U hCG, pregnyl; Merck Sharp & Dohme) was administered for final oocyte maturation when ≥ 3 follicles of ≥ 17 mm were observed. Transvaginal oocyte retrieval was performed under sedation 34–46 hours after hCG trigger. Retrieved metaphase II stage mature oocytes were fertillised by either IVF or ICSI, and embryo transfer was performed 48–72 hours after oocyte retrieval. Two embryos were transferred and serum beta-hCG was measured on Day 17 after embryo transfer to confirm the pregnancy. Women received Utrogestan (micronised progesterone; Besins Healthcare, Paris, France) 200 mg thrice daily for luteal phase support until serum beta-hCG was measured. Clinical pregnancy was defined as the presence of positive fetal cardiac activity and intrauterine gestation sac detection on ultrasonography at six weeks of pregnancy.
The primary objective of the study was to predict poor and excessive ovarian response to COH from individual biomarkers using serum AMH level, AFC, early FSH level, E2 level, BMI, ovarian volume and age. The secondary objective was to establish threshold values of ovarian markers in poor and excessive ovarian response to COH. The ovarian response was defined according to the number of oocytes retrieved. Poor ovarian response was defined as cancellation of cycles with > 2 follicles of > 11 mm in diameter, or < 4 oocytes retrieved at oocyte retrieval.(19) Excessive ovarian response was defined as > 19 oocytes retrieved during oocyte retrieval.(20)
Categorical variables were presented as numbers and percentages with 95% confidence interval (CI). Continuous variables were presented as mean ± standard deviation or median and range, depending on the distribution of data. To evaluate the significant independent predictors for poor and excessive responders, univariate and multivariate logistic regression with stepwise backward procedure was performed. The final model selection was carried out based on the likelihood ratio (LR) test. We also assessed the discrimination ability among logistic regression prediction models using net reclassification improvement (NRI) as well as indices of improvement in discrimination (IDI) statistics. The significance level was α = 0.05 in a two-sided test.
To compare the predictive abilities of different biomarkers for poor and excessive responders, receiver operating characteristic (ROC) curves were constructed to identify the global accuracy (area under the curve [AUC]) of our study covariates of interest (age, AFC, AMH and FSH level) based on predicted probability values, which was calculated using a post-estimation command after multivariate logistic regression with STATA statistical software Release 11 (StataCorp, College Station, TX, USA). The AUCs were estimated by points and 95% CI and compared using C-statistics.
Ideal cut-off points for age, AMH level, AFC and FSH level were determined using ROC curve and other statistics for any classification method. The ‘rocss’ command in STATA was used to calculate sensitivity and specificity using calculated probabilities from logistic regression analyses. Positive LR (LR+) and negative LR (LR−) were also calculated for all biomarkers to predict pre-specified clinical responses (poor and excessive responders). A two-sided p-value < 0.05 indicated statistical significance. A correlation matrix on all seven biomarkers was obtained with STATA.
RESULTS
A total of 263 women with a mean age of 35.0 ± 3.8 years were recruited for this study. The ethnicity of the study population was reflective of the demographics in Singapore, with the majority (76.4%) of the participants being Chinese. The main characteristics of this population, including their infertility diagnoses, are shown in
Table I
Demographic and clinical characteristics of the patients with oocytes retrieved during in vitro fertilisation (n = 263).
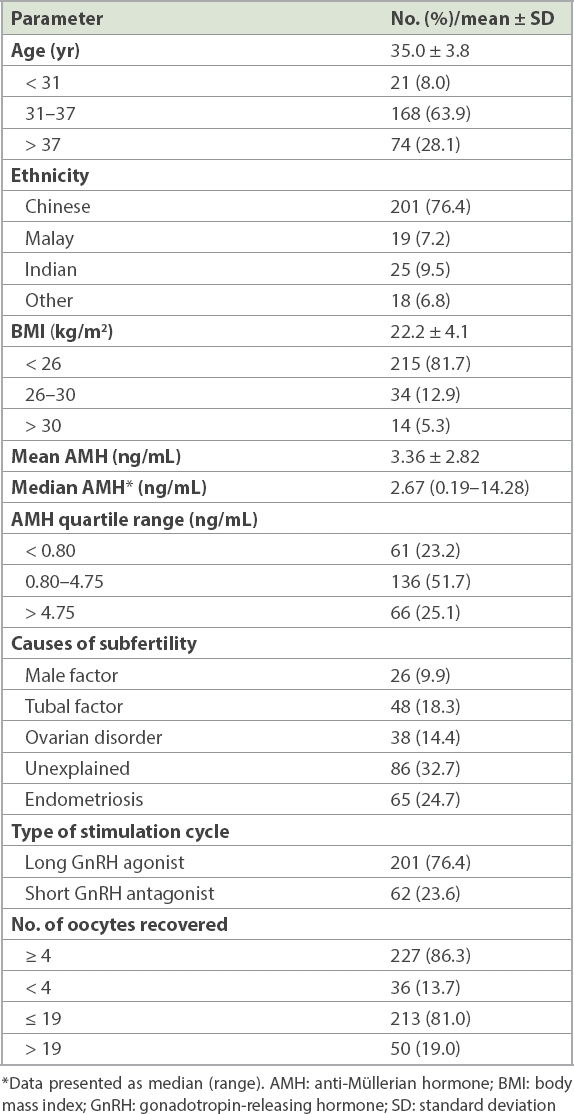
36 (13.7%) out of 263 women in the cohort had a poor response to COH with < 4 oocytes retrieved at oocyte retrieval, and 50 (19.0%) had an excessive response of ≥ 20 oocytes retrieved after COH. The AMH quartile range was < 0.80, 0.80–4.75 and > 4.75 for the < 25 percentile, 25–75 percentile and > 75 percentile ranges, respectively.
Univariate logistic regression analysis showed that only AMH level, AFC, Day 2/3 FSH level and age were independent significant predictors of poor response (
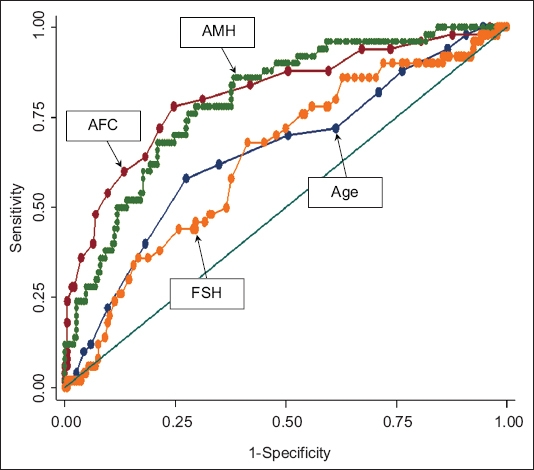
Fig. 1
Receiver operating characteristic curve shows the predictive ability of antral follicle count (AFC), anti-Müllerian hormone (AMH), age and follicle-stimulating hormone (FSH) for poor responders (based on probability calculated from univariate logistic regression).
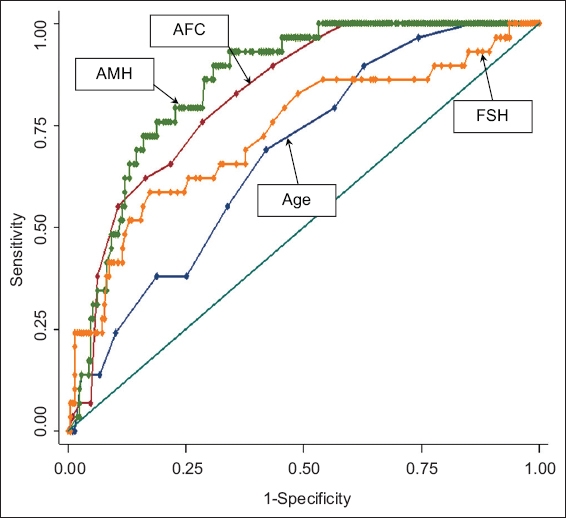
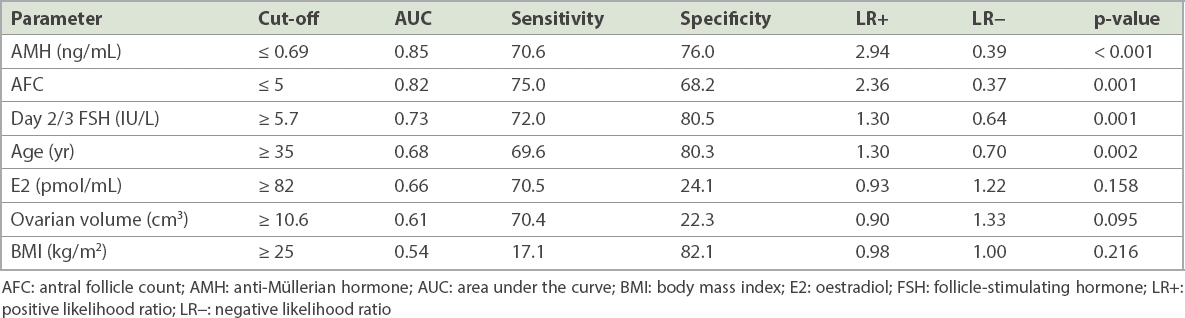
Table II
Univariate logistic regression analysis and sensitivity, specificity and likelihood ratios for the prediction of poor response at different clinical cut-off values.
AMH level, AFC, Day 2/3 FSH level and age were independent significant predictors for excessive ovarian response on univariate logistic regression analysis (
Table III
Predictors of hyper-responders using sensitivity, specificity and likelihood ratios according to clinical cut-off values, and the p-value of univariate logistic regression analysis.
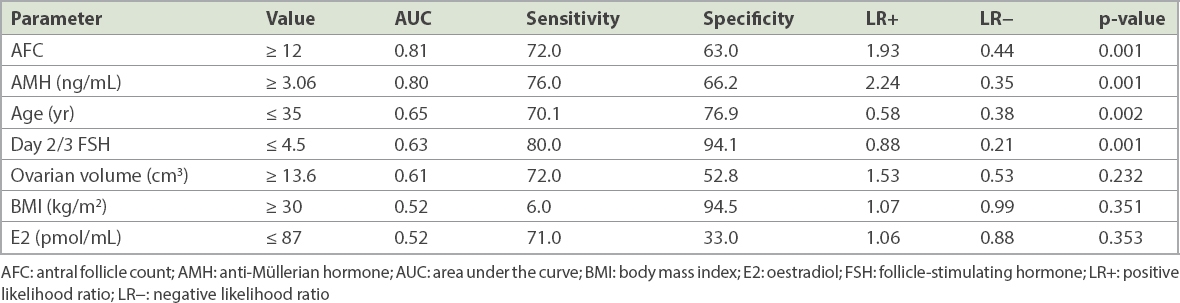
Fig. 2
Receiver operating characteristic curve shows the predictive ability of antral follicle count (AFC), anti-Müllerian hormone (AMH), age and follicle-stimulating hormone (FSH) for excessive responders (based on probability calculated from univariate logistic regression).
DISCUSSION
This prospective cohort study is one of a limited number of studies examining predictors of ovarian response in women undergoing IVF in a multi-ethnic Southeast Asian population in Singapore. Out of the seven possible markers, we found that AMH level and AFC are broadly equivalent in predicting both poor and excessive ovarian response in women undergoing IVF, in congruence with other studies published elsewhere.
ROC analyses showed that AMH level and AFC are the two better predictors of poor ovarian response compared to age, FSH level, E2 level, BMI and ovarian volume. Our finding that an AMH cut-off of ≤ 0.69 ng/mL and an AFC ≤ 5 were the best predictors for poor response is in broad agreement with a recent meta-analysis done by the IMPORT study group demonstrating the utility of using AMH level and AFC to predict poor ovarian response in women undergoing IVF treatment.(20) Our cut-off of ≤ 0.69 ng/mL (5 pmol/L) for the prediction of poor response is similar to those established by other groups.(21,22)
Reichman et al have also shown that an AMH level of < 0.7 ng/mL was associated with significantly higher cycle cancellation rates compared to an AMH level of > 2.01 ng/mL.(22) In contrast, a recent homogeneous Vietnamese cohort study by Lan et al suggested a higher cut-off of < 1.38 ng/mL to predict poor response (AUC 0.88). However, it is not known how AMH levels were measured in that study.(23) In our study, the corresponding value for prediction of poor response using AFC was ≤ 5, which was similar to that reported by Lan et al (AUC 0.82 vs. 0.80, respectively).(23) Thus, the high LRs associated with both AMH level and AFC suggest their clinical utility in predicting a poor response to COH.
To assess excessive response to COH, we selected an ovarian response of > 19 oocytes retrieved as a criteria for hyper-response, as this has been associated with a clinically important sixfold increase in moderate-to-severe OHSS at our centre for women who required admission (16.6% vs. 2.6%, unpublished data from 2008). Several other studies typically used the cut-off of > 15 oocytes.(20,24-26) Our findings closely mirror those from the IMPORT study group, where AMH level (AUC 0.80 vs. 0.81), AFC (AUC 0.81 vs. 0.79), FSH level (AUC 0.64 vs. 0.66) and age (AUC 0.65 vs. 0.61) were found to be useful predictors for excessive response (> 15 oocytes in the IMPORT study group).(27) In another study, Lee et al showed that an AMH cut-off level of 3.36 ng/mL is highly predictive for the development of OHSS in a Chinese population in Taiwan.(28) This level was similar to our cut-off of 3.06 ng/mL, although we did not use OHSS as an endpoint. Lan et al showed that both AMH level and AFC are good predictors for excessive response to COH at a lower cut-off value of 2.1 ng/mL for AMH (AUC 0.76) and a similar AFC cut-off of 12.25 (AUC 0.81).(23) Our data is also consistent with a recent local study by Lee et al showing that AMH level and AFC were reliable markers for prediction of ovarian response to COS, although the cut-off levels differ.(10) This may be due to the use of different definitions of poor ovarian response (≤ 4 oocytes vs. < 4 oocytes) and AFC measurements (2–10 mm vs. 2–9 mm) in their study compared to the present study.
The interpretation of AMH results may be affected by the various assays used. The first-generation AMH ELISA assay by Diagnostic Systems Laboratories (Webster, TX, USA) and Immunotech assay were replaced by the second-generation AMH Beckman Coulter, since 2011.(15,16,29) Our study was performed using the first-generation Diagnostic Systems Laboratories assay rather than the second-generation assays used in other studies, which may affect the comparison and interpretation of AMH readings. Recent publications have reported a discrepancy in AMH values obtained using the second-generation assay compared to those obtained with the Immunotech and Diagnostic Systems Laboratories assays.(29,30) The use of different assays should be taken into consideration when interpreting these measurements.
In our study, AMH levels and AFC results were not used to determine the dosage of FSH that patients received. Patients underwent IVF stimulation cycles based on their doctor’s clinical decision. The incorporation of AMH and/or AFC in tailoring IVF stimulation cycles may indeed allow an optimal ovarian response to be attained.(31,32) This allows us to tailor IVF stimulation regimens based on a patient’s AMH level, AFC and age to optimise an adequate response to COS and to prognosticate patients appropriately. As AFC and AMH levels decline over age, age-related nomograms can be used as a reference to tailor the ovarian stimulation regime in IVF.(11)
The ovarian reserve markers in our study had broadly similar measurements across the different ethnic groups in our study. However, we acknowledge that the small number (n = 62) of patients from minority ethnic groups limited our ability to draw conclusions about interethnic group differences.
In conclusion, AMH level and AFC are good predictors of ovarian response in Southeast Asian women undergoing IVF in Singapore. We did not find BMI, ovarian volume and early follicular phase E2 level to be useful predictors of ovarian response to COH. The observed differences in poor and excessive responders with other published studies suggest that centre- and population-specific values should be used to prognosticate patients for IVF stimulation regimens.
ACKNOWLEDGEMENT
Chan JKY received salary support from the National Medical Research Council (NMRC/CSA-SI/008-2016), Ministry of Health, Singapore.


