Abstract
Right ventricle to pulmonary artery (RV-PA) conduits have been used for the surgical repair of congenital heart defects. These conduits frequently become stenosed or develop insufficiency with time, necessitating reoperations. Percutanous pulmonary valve implantation (PPVI) can delay the need for repeated surgeries in patients with congenital heart defects and degenerated RV-PA conduits. We presented our first experience with PPVI and described in detail the procedural methods and the considerations that are needed for this intervention to be successful. Immediate and short-term clinical outcomes of our patients were reported. Good haemodynamic results were obtained, both angiographically and on echocardiography. PPVI provides an excellent alternative to repeat open-heart surgery for patients with congenital heart defects and degenerated RV-PA conduits. This represents a paradigm shift in the management of congenital heart disease, which is traditionally managed by open-heart surgery.
INTRODUCTION
Percutaneous pulmonary valve implantation (PPVI) was first conceptualised by Bonhoeffer et al in 2000,(1) providing an alternative treatment option to open surgical replacement for patients with congenital heart disease and degenerated right ventricle to pulmonary artery (RV-PA) conduits. Valved conduits consist of synthetic material or homograft/xenograft tissue. Their disadvantages are the inability to keep pace with the growth of the patient, mechanical distortion, and progressive degeneration leading to conduit stenosis over time. Regurgitation may also occur as the valve leaflets degenerate. These patients often require multiple sternotomies throughout their lifespan because the surgical conduits have limited durability, necessitating ongoing intermittent replacement. Each redo cardiac surgery is associated with higher technical operative difficulty and becomes increasingly hazardous, with greater mortality and morbidity risks.(2,3) PPVI has been shown to extend the duration of these conduits, thus reducing the total burden of redo surgeries for these patients.
For this study, we selected our patients with close attention to the indications and contraindications for this procedure, listed in
Box 1
Selection criteria for percutaneous pulmonary valve implantation among patients with right ventricle to pulmonary artery conduit obstruction:
In the case of RV-PA conduit obstruction, the efficacy of PPVI on clinical outcome is well-established,(6,7) and the European Society of Cardiology guidelines(4) are precise in recommending the timing for early intervention. On the other hand, pulmonary regurgitation has been well tolerated for many years and the impact of early PPVI in these patients is more controversial.(5,7) It has been suggested that the degree of right ventricular dilation should prompt the PPVI indication, rather than the severity of the pulmonary regurgitation.(8)
The Melody™ transcatheter pulmonary valve (Medtronic Inc, Minneapolis, MN, USA), the first commercially available transcatheter valve in the world, received European certification and Health Canada approval in 2006. It is a bioprosthetic valve harvested from a bovine jugular vein and sutured within a balloon-expandable platinum-iridium stent. The valve size is 18 mm in diameter, which is crimped to 6 mm and can then be balloon-expanded to three sizes – 18 mm, 20 mm and 22 mm – depending on the balloon on which it is mounted. The Melody valve is the most commonly used valve in the world in the pulmonary position, and most of the available data for PPVI is from its use.(9)
In this case series, we present our first experience with PPVI using the Melody valve for patients with RV-PA conduit stenosis in Singapore. The procedural methods and the considerations needed for a successful intervention are described in detail. The immediate and short-term clinical outcomes of our patients for whom PPVI was performed are also reported.
METHODS
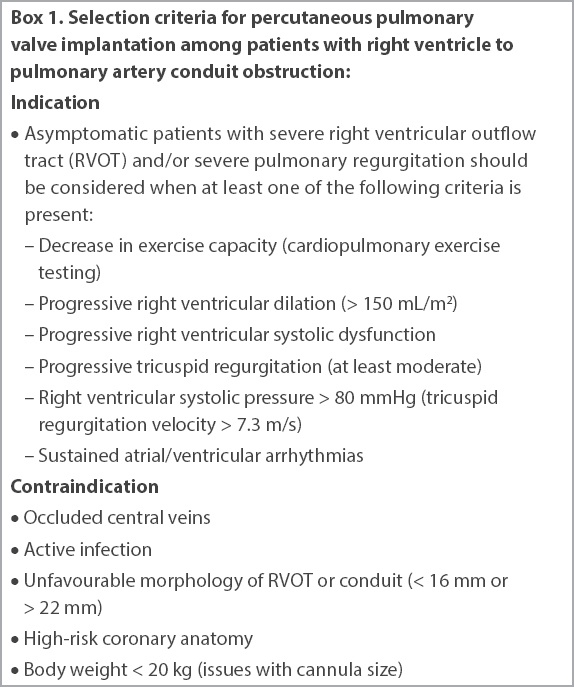
The Melody valve was implanted through a dedicated delivery system (Ensemble™ Transcatheter Delivery System, Medtronic Inc, MN, USA) consisting of a balloon-in-balloon system that allows repositioning during the valve delivery (
Fig. 1
Photographs show the (a) Melody valve and (b) Ensemble Transcatheter Delivery System.
Careful consideration was essential to select the appropriate patients from the cohort of adult congenital heart patients followed at our institution. Investigations such as transthoracic echocardiography, cardiac magnetic resonance imaging, computed tomography (CT) angiography, cardiopulmonary exercise test and right heart catheterisation were performed for preprocedural patient screening and assessment. A dedicated heart team comprising paediatric cardiologists, adult cardiologists, imaging specialists and congenital cardiothoracic surgeons made the joint decisions on eligibility for PPVI and procedural planning.
Femoral venous access was obtained percutaneously and haemodynamic assessment was performed to assess conduit stenosis and severity of RV-PA gradient and/or pulmonary regurgitation. Angiography of the right ventricular outflow tract (RVOT) and conduit was performed for various outflow tract measurements. A 14-Fr 75-cm long Mullins sheath (Cook Medical, Bloomington, IN, USA) was placed with its tip just distal to the conduit over a 260-cm long stiff guide wire, such as a Lunderquist wire (Cook Medical). A noncompliant balloon was then inflated within the landing zone of the RV-PA conduit to recheck measurements, and simultaneous left coronary angiography was performed to assess the risk of coronary artery compression. This test step was essential, as inflating the balloon during stent or valve implantation may cause extrinsic compression of the coronary artery if it is in close proximity. After this, the conduit was pre-stented with bare or covered stents, depending on whether calcification was present. This was an improved procedural modification over the initial cases of Melody valve implantation. The additional step of pre-stenting the conduit conveyed radial strength and reduced tension on the Melody valve, thereby reducing the risk of fracture of the valve stent. Importantly, as conduits develop significant calcification over the years, covered stents act as a safety harness in case of conduit rupture or dissection during dilation. After pre-stenting the conduit, any residual narrowing can be addressed by repeated balloon dilatation using a higher pressure noncompliant balloon. Thereafter, the Melody valve was implanted within this pre-stented scaffold. Invasive haemodynamic assessments were then performed using a multi-track catheter (NuMED Inc, Hopkinton, NY, USA) and final angiography was performed to assess the results.
CASE SERIES
Patient 1
A 31-year-old man with Tetralogy of Fallot had undergone initial surgical repair at four years of age and RV-PA conduit insertion using a 21-mm pulmonary homograft 13 years later. This was followed seven years later by repeat open-heart surgery for homograft re-replacement using an aortic homograft of 20 mm. The third open-heart surgery was complicated by severe bleeding from adhesions and a stormy recovery requiring prolonged stay in the intensive care unit. Subsequently, the patient was found to have progressive elevation of the RVOT pressure gradients from 25 mmHg to 44 mmHg over five years. He became symptomatic with complaints of lethargy and palpitations, requiring treatment with oral amiodarone for episodes of supraventricular tachycardia. Cardiac magnetic resonance imaging performed six months prior to PPVI showed moderate homograft stenosis and mild pulmonary regurgitation. The mean right ventricular end-diastolic volume was 160 mL/m2. Right ventricular ejection fraction was reduced to 41%. The most recent transthoracic echocardiography (three weeks prior to PPVI) showed an estimated right ventricular systolic pressure of 78 mmHg, with severe right ventricular dysfunction and moderate tricuspid regurgitation. His cardiopulmonary exercise test showed a reduced peak VO2 max of 18 mL/kg/minute (33% of predicted). With these criteria, the patient fulfilled the indications for PPVI.
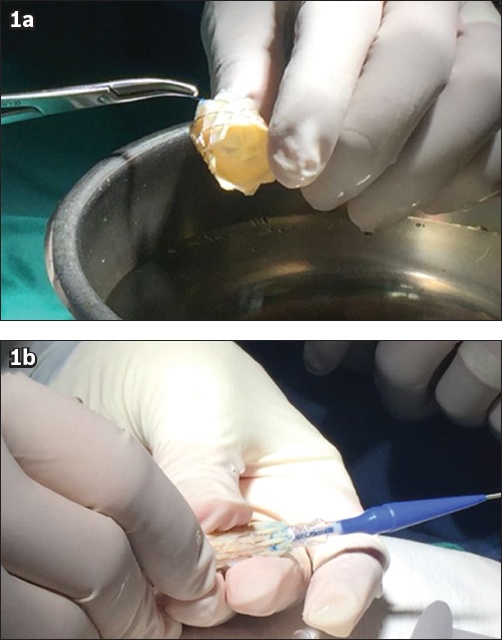
The procedure was performed under general anaesthesia. Two ProGlide sutures were used to preclose the femoral venous access, which was sequentially dilated to permit the placement of a 14-Fr 75-cm long Mullins sheath. Pretreatment peak-to-peak gradient was 25 mmHg under general anaesthesia and in the presence of reduced right ventricular function. Initial angiographic measurements were performed to assess the conduit size as well as to ensure no compromise of the coronary artery (Figs.
Fig. 2
Patient 1: Angiographic projections in the (a & c) anteroposterior and (b & d–h) lateral views during a procedure show (a & b) measurements of the conduit; (c) balloon inflation for assessment of the conduit and coronary artery interaction; pre-stenting, with (d) placement and (e) deployment of the covered stent; (f) post-dilatation of the covered stent with the Atlas balloon; and implantation, with (g) placement and (h) deployment of the Melody valve. AP: anteroposterior; Lat: lateral
The patient was treated with aspirin 100 mg once daily and clopidogrel 75 mg once daily for one month and subsequently planned for lifelong aspirin. He was also given intravenous cefazolin perioperatively and five days of oral augmentin. Although transthoracic echocardiography two days after PPVI showed a similar RVOT peak gradient of 40 mmHg (no change from preprocedure echocardiography), right ventricular systolic dysfunction had improved remarkably from severe to mild. The preprocedure RV-PA gradient had probably been underestimated due to severe right ventricular systolic dysfunction. There was no pericardial effusion, and the leaflets were functioning well without pulmonary regurgitation. The patient was discharged well on the fifth postoperative day.
Patient 2
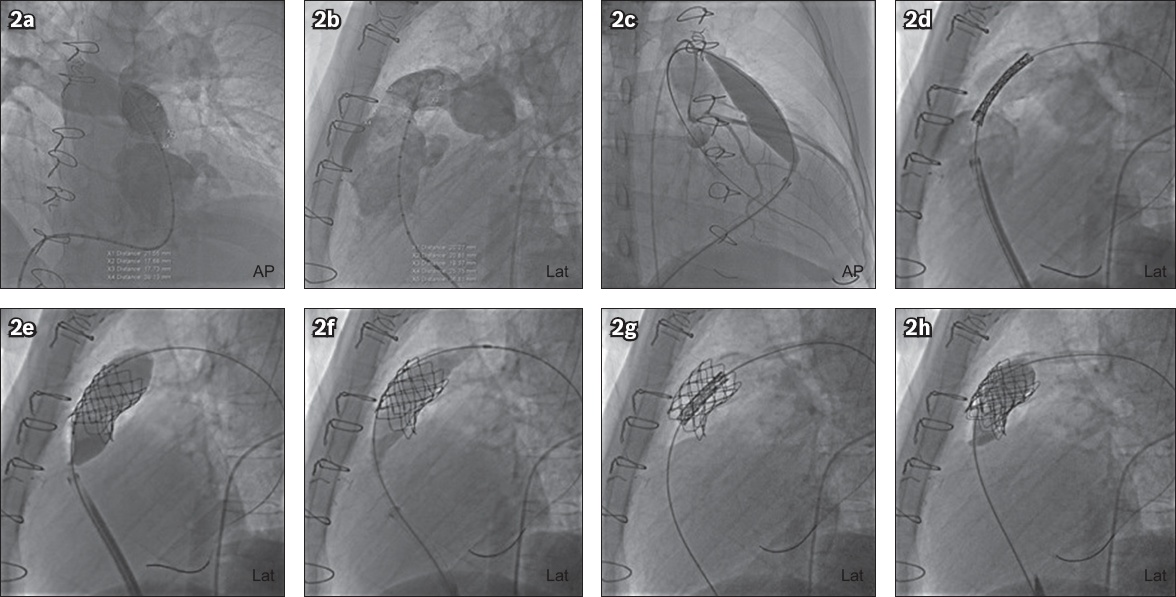
A 21-year-old man with truncus arteriosus Type 1 not associated with DiGeorge syndrome underwent a Rastelli operation with closure of the ventricular and atrial septal defects and division of patent ductus arteriosus at 29 days of age. A redo cardiac surgery was performed two years later, involving a 17-mm homograft revision and right pulmonary artery angioplasty (
Fig. 3
Patient 2: CT images show (a) anatomy of the conduit post-Rastelli repair for truncus arteriosus; (b) the calcified conduit (length 2.97 cm); and (c) relationship between the left coronary artery (arrow) and the conduit. (d) Transthoracic echocardiogram shows high right ventricular outflow tract (RVOT) pressure gradients before Melody valve implantation. Fluoroscopy images show (e) pre-stenting, with placement of a second covered stent; and implantation, with (f) placement and (g) deployment of the Melody valve, while transthoracic echocardiogram shows (h) improvement in RVOT pressure gradients after Melody valve implantation.
During pre-procedural planning, extensive calcification of the conduit was noted on cardiac CT (
Transthoracic echocardiography performed the next day after PPVI showed an improvement of 40 mmHg in RVOT peak gradient, to 25 mmHg (
DISCUSSION
To the best of our knowledge, this is the first experience of transcatheter PPVI for patients with congenital heart defects and degenerated RV-PA conduits in Singapore.
Given the overall improved life expectancy of patients with surgically corrected congenital heart defects over the decades,(10) the role of interventional therapy such as PPVI should be expected to expand, as this cohort continues to survive longer and grow in number. The short-term safety profile of the procedure is well established. The mid-term data has also been reassuring, with the recent publication of an up-to-seven-year follow-up of the Melody valve that had a median follow-up time of 4.5 years.(11) Risks for such procedures, however, persist and include: (a) conduit rupture; (b) coronary artery compression/occlusion; (c) infective endocarditis; and (d) valve stent fracture.
Partial or total conduit rupture may follow balloon predilation and are especially likely to occur in very stenotic and highly calcified conduits. The risk of conduit rupture requiring rescue surgery can be up to 2%.(12,13) We used covered stents in our patients to mitigate against this risk, so that any extravasation due to conduit rupture may be confined. Most of the time, this complication of conduit rupture may be managed in the catheterisation laboratory.(14,15)
Coronary artery compression or occlusion can be fatal, as it may lead to acute myocardial infarction and occurs in about 1% of Melody valve implantations.(12,13) Evaluation with prior CT coronary angiography and balloon testing during the procedure is useful for identifying high-risk cases, allowing timely preventive measures to be taken. The incidence of coronary artery compression during balloon testing ranges from 4.7% to 6%.(16)
There has been some recent concern over the rate of infective endocarditis reported post PPVI.(12,13) To date, the estimated annualised rate of a first episode of infective endocarditis is 2.4% per patient-year.(17) This could be related to slow flow and residual turbulence in the conduits. There could also be occult infection in the previous homograft/conduit for which the Melody valve was implanted. Infection rates of the Contegra conduit were reported to be as high as 11.3% (12 out of 106 patients) in those implanted, at a median follow-up of 4.4 years.(18) It is important to emphasise that for these patients, antibiotic prophylaxis and good dental hygiene are of utmost importance to reduce the risk of subsequent endocarditis.(19)
It has been reported that valve stent fracture can be mitigated by modifying the procedure, adopting systematic pre-stenting of the conduit.(20) This complication occurred at a rate of about 20% in earlier series,(21) which has been reduced to 5%–16% after pre-stenting.(12,13) Melody valve stent fractures are generally well tolerated but can potentially lead to the loss of structural integrity, embolisation or restenosis.(22)
After the PPVI procedure, it is recommended to start patients on dual antiplatelet therapy for one month and subsequent lifelong aspirin. In the immediate follow-up period, the patient should also be advised to report any new-onset arrhythmias or worsening of pre-existing arrhythmia. The incidence of post-implant arrhythmias was reported to be 15% in a recent study, but a majority of these arrhythmias were resolved by six months on follow-up.(23) Finally, it is imperative that patients are educated on the need for antibiotic prophylaxis and encouraged to be meticulous in maintaining good dental hygiene.
The longevity of surgical RV-PA conduits is known, but long-term data with PPVI is relatively scarce. Around 50% of RV-PA conduits require replacement within ten years.(5) Subsequent conduits may have shorter survival than the original.(24) PPVI allows concomitant relief of RVOT obstruction and regurgitation while maintaining pulmonary valve competence.(14) At five years after PPVI, 76% of patients remain free from reintervention and 92% of patients remain free from explant.(11)
The risks described above and the longevity of the valve implant have to be taken into careful consideration during patient selection, weighing them against the increased operative risks associated with redo open-heart surgery. Generally, the overall lower procedure-related morbidity, together with other advantages such as a shorter hospital stay, less pain and faster recovery, make PPVI an attractive alternative to repeated sternotomies.
In conclusion, PPVI provides an excellent alternative to open-heart surgery in patients with congenital heart disease and degenerated RV-PA conduits. The importance of heart team collaboration, detailed preprocedural assessment and postprocedural care, especially for the prevention of infective endocarditis post PPVI, cannot be overemphasised.


