Abstract
INTRODUCTION
Percutaneous transcatheter aortic valve implantation (TAVI) has become an established therapy for inoperable and high-surgical-risk patients with severe aortic stenosis. Although TAVI in patients with degenerated surgical aortic bioprostheses (i.e. valve-in-valve TAVI) is increasingly reported in Western studies, such data is lacking in Asian patients. We describe the initial experience of valve-in-valve TAVI in Asia.
METHODS
Eight patients who underwent valve-in-valve TAVI due to degenerated aortic bioprostheses were enrolled. The mechanism of bioprosthetic valve failure was stenotic, regurgitation or mixed. All procedures were performed via transfemoral arterial access, using the self-expanding CoreValve prosthesis or balloon-expandable SAPIEN XT prosthesis.
RESULTS
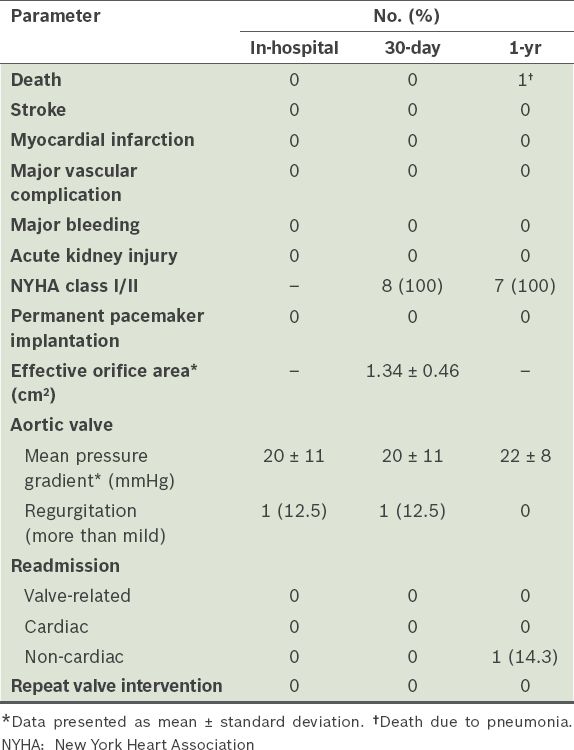
The mean age of the patients was 71.6 ± 13.2 years and five were male. Mean duration to surgical bioprosthesis degeneration was 10.2 ± 4.1 years. Valve-in-valve TAVI was successfully performed in all patients. CoreValve and SAPIEN XT prostheses were used in six and two patients, respectively. There were no deaths, strokes or permanent pacemaker requirement at 30 days, with one noncardiac mortality at one year. All patients experienced New York Heart Association functional class improvement. Post-procedure mean pressure gradients were 20 ± 11 mmHg and 22 ± 8 mmHg at 30 days and one year, respectively. Residual aortic regurgitation (AR) of more than mild severity occurred in one patient at 30 days. At one year, only one patient had mild residual AR.
CONCLUSION
In our experience of valve-in-valve TAVI, procedural success was achieved in all patients without adverse events at 30 days. Good clinical and haemodynamic outcomes were sustained at one year.
INTRODUCTION
Percutaneous transcatheter aortic valve implantation (TAVI), also known as transcatheter aortic valve replacement, has become the treatment of choice for inoperable patients with severe aortic valve stenosis(1,2) and an alternative treatment to open chest surgical aortic valve replacement (SAVR) in patients at high surgical risk.(3,4) Due to the minimally invasive nature of the technique, valve-in-valve TAVI is increasingly used as an alternative treatment modality in patients with degenerated surgical bioprostheses in the aortic position.
Valve-in-valve TAVI is an attractive option for patients with a previous aortic surgical bioprosthesis; these patients are considered to be at elevated risk for reoperation due to various reasons, including technical difficulties of a scarred chest, advanced patient age and other comorbidities. Several case series, including a large global registry on valve-in-valve TAVI,(5-9) have been reported. However, such data on Asian patients is lacking. We describe the first case series of valve-in-valve TAVI for degenerated aortic surgical bioprostheses in Asia.
METHODS
Eight consecutive patients who underwent valve-in-valve TAVI in a single centre due to degenerated surgical bioprostheses in the aortic position were included in the study. All patients were evaluated by a multidisciplinary heart team, including two cardiac surgeons, and were deemed to be at elevated risk of redo open heart surgery. Bioprosthetic aortic valve failures were categorised, based on the primary mechanism of failure, into stenotic, regurgitation or mixed (with at least a moderate degree of both stenosis and regurgitation), according to the recommendations of the American Society of Echocardiography.(10)
All valve-in-valve TAVI procedures were performed using either the self-expanding CoreValve (Medtronic Inc, Minneapolis, MN, USA) prosthesis (
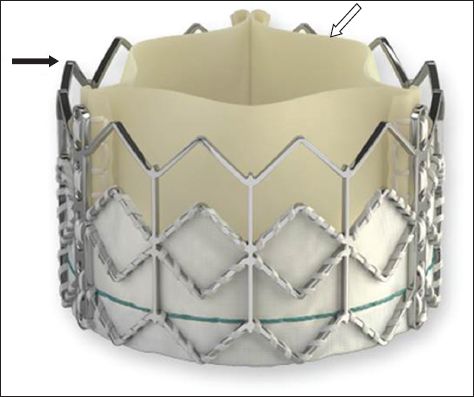
Fig. 1
Photograph shows the self-expanding CoreValve transcatheter heart valve with a nitinol frame (black arrow) and porcine pericardial leaflets (white arrow).
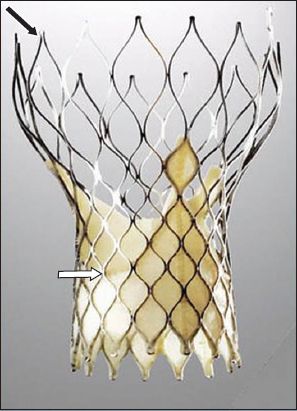
Fig. 2
Photograph shows the balloon-expandable SAPIEN XT transcatheter heart valve with a cobalt chromium frame (black arrow) and bovine pericardial leaflets (white arrow).
Patient characteristics, details of previous surgical bioprostheses, and procedural and clinical outcomes were recorded. Procedural success and all adverse clinical outcomes and events were defined according to the Valve Academic Research Consortium-2 consensus document.(11) All patients gave their informed consent for the study.
RESULTS
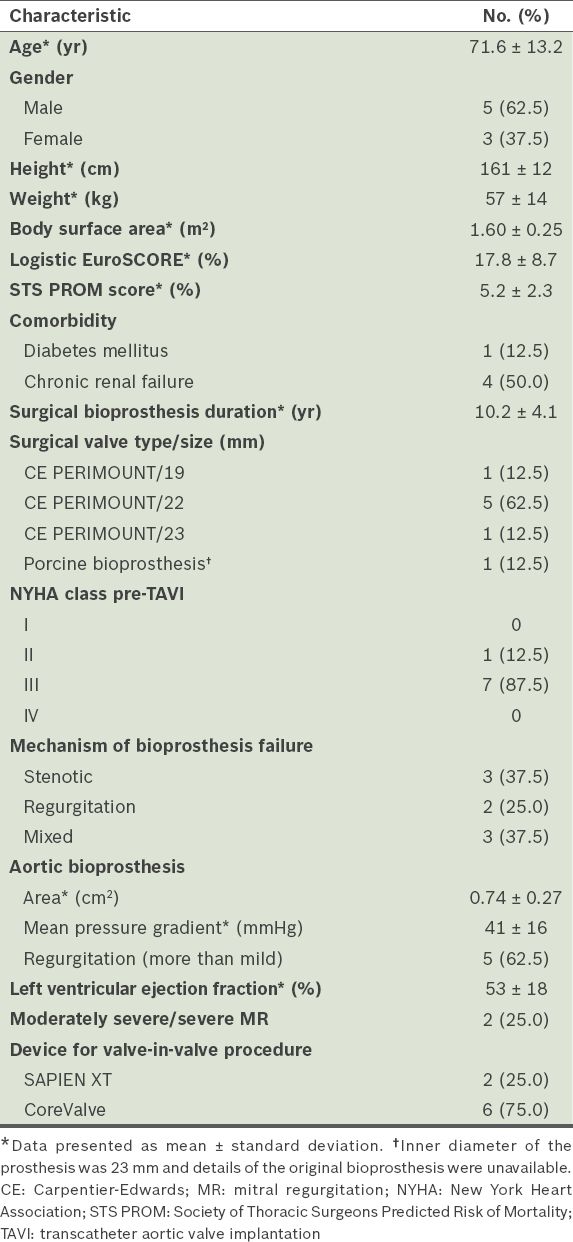
The mean age of the eight patients was 71.6 ± 13.2 years and 5 (62.5%) were male. Their baseline characteristics are summarised in
Table I
Baseline characteristics of the patients (n = 8).
Valve-in-valve TAVI was successfully performed in all patients via the transfemoral approach. The CoreValve prosthesis (Figs.
Fig. 3
Photograph shows a self-expanding CoreValve transcatheter heart valve implanted within a surgical bioprosthetic valve. The CoreValve frame (arrow) is seen.

Fig. 4
Fluoroscopic images show (a) a Carpentier-Edwards PERIMOUNT surgical aortic valve bioprosthesis (black arrow) with (b) a self-expanding CoreValve transcatheter heart valve implanted within (white arrows indicate CoreValve frame).
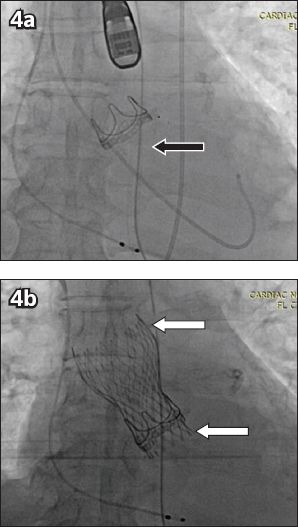
Fig. 5
Photograph shows a balloon-expandable SAPIEN XT transcatheter heart valve implanted with a surgical bioprosthetic valve (arrows indicate SAPIEN XT frame).
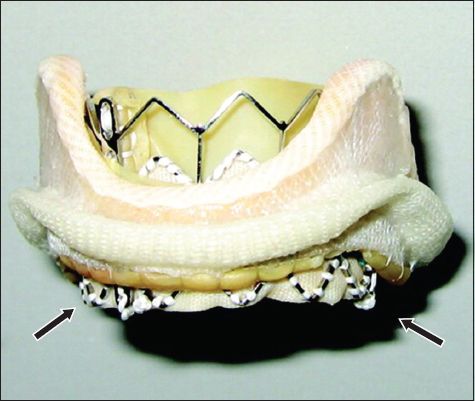
Fig. 6
Fluoroscopic images show (a) a Carpentier-Edwards PERIMOUNT surgical aortic valve bioprosthesis (black arrow) with (b) a balloon-expandable SAPIEN transcatheter heart valve implanted within (white arrow indicates SAPIEN XT frame).
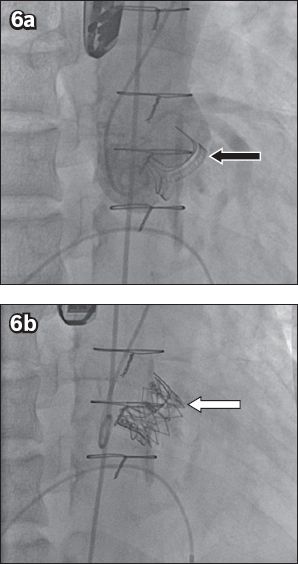
Table II
Haemodynamic and clinical outcomes (n = 8).
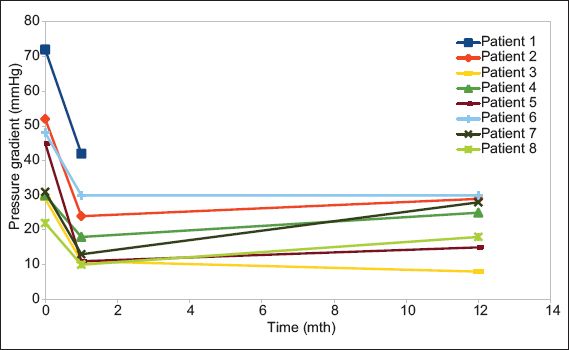
The overall survival rate at one year was 87.5% (n = 7). The only mortality, which was due to pneumonia, occurred in a patient who had end-stage renal failure on haemodialysis at 11 months after the procedure. For the rest of the patients, post-procedure pressure gradients were maintained at 30 days and one year, with a post-procedure mean pressure gradient (MPG) of 20 ± 11 mmHg and 22 ± 8 mmHg, respectively (
Fig. 7
Line graph shows the changes in mean pressure gradient in the eight patients at baseline, 30 days and one year.
DISCUSSION
Bioprosthetic valves are increasingly used in preference to mechanical valves in open SAVR, as lifelong oral anticoagulation is not required and its attendant complications can be avoided. A recent study has shown similar long-term survival rates in patients with bioprosthetic and mechanical valves. However, patients with bioprosthetic valves require reoperations due to their limited durability and expected degeneration;(12) those undergoing such reoperations face increased surgical risk due to their more advanced age (compared with their age during the index SAVR) during the redo surgery and scarring in the non-virgin chest. Consequently, valve-in-valve TAVI appears to be an attractive, minimally invasive method of replacing the degenerated surgical aortic bioprosthesis without the need for a reoperation. Several small case series and a global registry have shown that it is a feasible and viable option for patients at high risk.(5-9)
In our initial Asian experience of valve-in-valve TAVI in eight patients with degenerated bioprostheses in the aortic position, all procedures were successful, with no mortality or stroke event at 30 days, an 87.5% survival rate at one year, and a single mortality due to a noncardiac cause. The results compare favourably with those of previous studies, which had 30-day mortality rates of 0%–17%, stroke rates of 0%–2%, and a one-year survival rate of 85.8%.(5-9) Although the majority of our patients (75.0%) had small surgical bioprostheses, the post-procedure 30-day MPG was 20 mmHg, which is similar to that reported in the global registry (mean 16.0 [interquartile range 9.1] mmHg).(8) This is, however, higher than the average expected post-procedure MPG (10 mmHg) for TAVI in a native aortic valve.(1-4)
In the present study, the three patients who had a significant post-procedure residual MPG > 20 mmHg previously had small surgical bioprostheses. The three patients with severe PPM also had small surgical bioprostheses (≤ 21-mm PERIMOUNT). The most likely explanation is that these patients already had a high residual MPG and PPM after the initial surgical bioprosthetic valve replacement.(13) Although the TAVI procedure can significantly reduce the pressure gradient, it cannot overcome high baseline pressure gradients or baseline PPM. Larger surgical bioprostheses implanted during the index procedure would reduce baseline PPM and result in better pressure gradients when a valve-in-valve TAVI procedure becomes necessary. Although none of our patients with high residual pressure gradients or PPM had any cardiac events at one year, a longer follow-up period was needed to ascertain the clinical impact of PPM.
In the global registry, a residual pressure gradient > 20 mmHg was found in 27% of patients, although small (≤ 21 mm labelled size) surgical bioprostheses accounted for only 29% of their total population.(8,9) Interestingly, the global registry also observed that the CoreValve prosthesis resulted in a lower pressure gradient in small surgical bioprostheses, compared to the balloon-expandable valve, due to its supra-annular valve design.(8,9) Our series is too small to draw a similar conclusion; however, based on the global registry experience, the CoreValve prosthesis is currently preferred for aortic valve-in-valve TAVI, particularly if the surgical bioprosthesis is ≤ 21 mm in size.
Only one patient in our series had moderate residual AR at 30 days, which became mild at one year. This patient received a self-expanding transcatheter valve, and it is likely that the continued outward expansion of the frame resulted in better sealing (and thus decreased paravalvular AR) over time. The other surviving patients had none or trivial residual AR. Similarly, this result compares favourably with the global registry, where only 5% of patients had more than mild residual AR.(8,9) The low rate of post-procedure AR, as compared to native valve TAVI, is likely due to the fact that the surgical bioprosthetic ring is circular and the sewing ring is not heavily calcified, allowing for good apposition of the new TAVI prosthesis within the bioprosthetic ring. This is an important finding, as even mild paravalvular AR after TAVI has been shown to result in reduced longer-term survival globally, as compared to patients with no or trivial paravalvular AR following TAVI in native aortic valves.(3,14)
One limitation of this study is that it was a small retrospective study among a group of highly selected patients. Thus, the results cannot be generalised to all valve-in-valve TAVI procedures in Asian patients. Furthermore, as nearly all our patients were implanted with a single model of surgical bioprosthesis, results may differ in patients with other types of surgical aortic bioprostheses.
In conclusion, we reported the initial Asian experience of valve-in-valve TAVI for degenerated surgical aortic bioprostheses. Valve-in-valve TAVI was successfully performed in eight patients without adverse events at 30 days; good clinical and haemodynamic outcomes were sustained at one year. Valve-in-valve TAVI appears to be a feasible, relatively safe and less invasive alternative therapy for Asian patients with degenerated surgical aortic valve bioprostheses. At the initial SAVR, the largest-possible bioprosthesis should be implanted (> 21 mm labelled size) to allow for a better haemodynamic profile if a subsequent valve-in-valve TAVI becomes necessary. These findings may provide an impetus to employ more bioprostheses, rather than mechanical valves, in younger patients undergoing open SAVR in the near future, given that this effective technique of replacing a failed surgical bioprosthesis is available.


