Abstract
INTRODUCTION
Linkage to care among individuals with substance misuse remains a barrier to the elimination of the hepatitis C virus (HCV). We aimed to determine whether point-of-care (PoC) education, screening and staging for liver disease with direct access to hospitals would improve linkage to care among this group.
METHODS
All participants were offered PoC education and HCV screening. HCV-positive participants were randomised to standard care (controls) or direct access, which provided a direct pathway to hospitals. Linkage to care was determined by reviewing electronic medical records. Linkage of care cascade was defined as attendance at the specialist clinic, confirmation of viraemia by HCV RNA testing, discussion about HCV treatment and initiation of treatment.
RESULTS
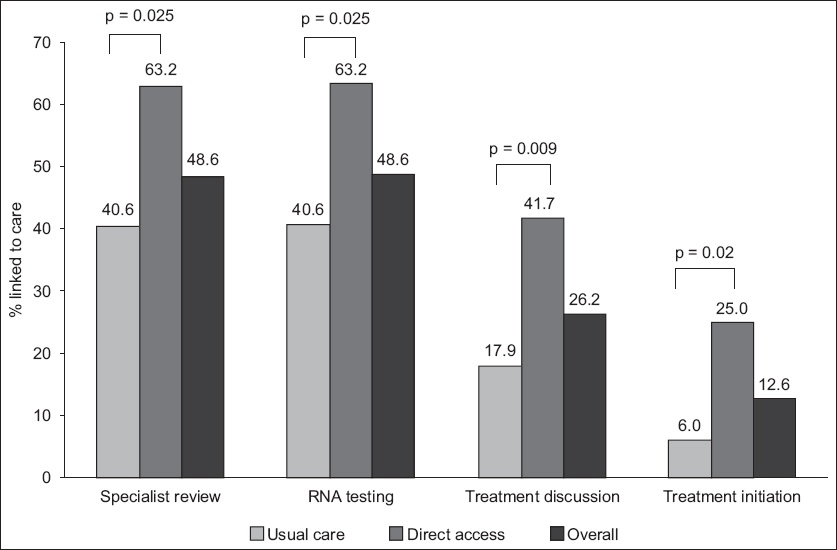
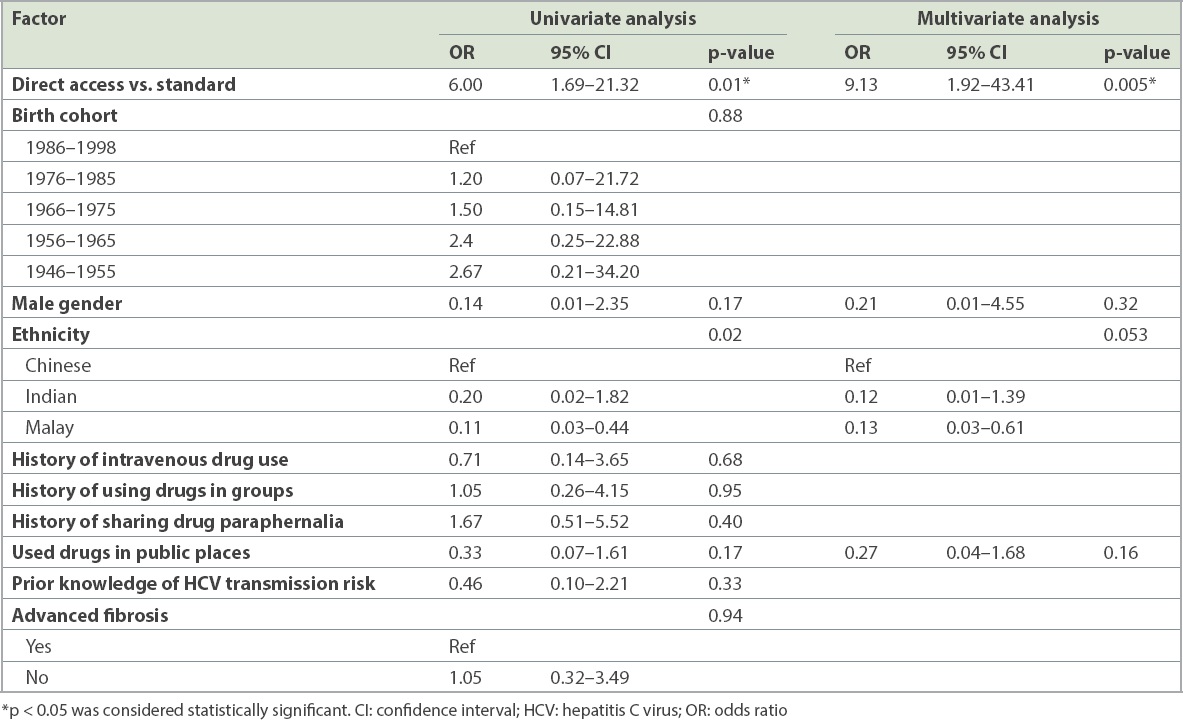
351 halfway house residents were screened. The overall HCV prevalence was 30.5% (n = 107), with 69 residents in the control group and 38 in the direct access group. The direct access group had a significantly higher percentage of cases linked to specialist review for confirmatory RNA testing (63.2% vs. 40.6%, p = 0.025), HCV treatment discussion (p = 0.009) and treatment initiation (p = 0.01) compared to the controls. Overall, only 12.6% (n = 13) had treatment initiation during follow-up. PoC HCV screening with direct access referral had significantly higher linkage to HCV treatment initiation (adjusted odds ratio 9.13, p = 0.005) in multivariate analysis.
CONCLUSION
PoC HCV screening with direct access improves linkage to care and simplifies the HCV care cascade, leading to improved treatment uptake. PoC education, screening, diagnosis and treatment may be an effective strategy to achieving HCV micro-elimination in this population.
INTRODUCTION
The hepatitis C virus (HCV) is a major global public health concern, with more than 71 million individuals estimated to be infected worldwide.(1) HCV is a leading cause for liver transplantation in the Western world and continues to be an important cause of decompensated cirrhosis.(2,3) With the recent advent of direct-acting antivirals (DAAs), the landscape of HCV treatment has seen a radical change. However, DAA treatment remains inaccessible and expensive in many parts of the world. The World Health Organization (WHO) has declared an urgent need to eliminate viral hepatitis globally by the year 2030.(4) Such strategies require coordinated and concerted effort from government-based agencies and healthcare organisations.(5,6) Case finding and linkage to care are pivotal aspects of any HCV elimination strategy.
Strategic planning of HCV elimination in Singapore has been hampered by a paucity of epidemiological data that accurately assesses the scale of the problem. The prevalence of HCV in people who inject drugs (PWID) is estimated to be between 42% and 62%.(6) Estimates from Singapore have shown that the figure is as high as 42.5% among a small cohort of PWID at a community addiction programme.(7) In Singapore, halfway houses are interim accommodations provided to released prisoners with recent incarcerations for substance misuse or voluntary attendees seeking drug rehabilitation. Each year, about 500–700 people are placed into halfway houses for community-based drug rehabilitation programmes, which provide interim job allocation and training to facilitate their reintegration into society. The residents typically stay at halfway houses for 6–12 months. Therefore, halfway houses provide a stable population of individuals with a high prevalence of HCV and are ideal for case finding and treatment.
Linkage to care is a vital step in the care cascade of HCV elimination. It is typically poor among individuals with substance misuse. Barriers include psychosocial factors such as stigma, lack of awareness, inefficient screening and limited access to healthcare.(8) Given the anticipated high HCV prevalence at halfway houses, there is a need for education on the risk of HCV transmission, HCV screening and a simplified linkage-to-care cascade. Currently, data on interventions to improve the HCV care cascade is lacking, and strategies are required in order to achieve HCV elimination targets by the year 2030.(9) We herein examined the feasibility of a decentralised point-of-care (PoC) HCV model of education, screening and facilitated ‘direct access’ referrals of HCV-positive cases. The primary aim was to determine whether decentralised HCV education and screening within halfway houses with subsequent direct access referrals would improve HCV case detection, linkage of care and treatment uptake among the drug misuse population. We propose that such a model of care would improve the rate of retention within the HCV care cascade.
METHODS
Halfway house residents in Singapore are a population of ex-drug users who were recently released from prison. We recruited patients aged 21 years and above from participating halfway houses in Singapore between February 2017 and September 2018. The recruitment was spread over an 18-month period with staggered visits, as the typical turnover of residents in halfway houses was 6–12 months. We visited ten of the 12 halfway houses in Singapore at least once to obtain a representative sample of halfway house residents across the country. The majority of halfway houses in the study were within a 10-km radius of Changi General Hospital (CGH), with the furthest located 25 km away. This study was approved by the SingHealth Centralised Institutional Review Board, Singapore, and registered on the ClinicalTrials.gov website (identifier NCT03566563).
All participants were offered PoC education and HCV screening. Universal HCV 45-minute education sessions were provided by the principal investigators (Thurairajah PH or Hsiang JC) to halfway house residents. The educational session included information on the risk factors of HCV transmission, liver disease progression, complications from HCV infections and treatment options, as well as a short motivational talk on reducing high-risk behaviour. A team of three nurses and the investigators provided the subsequent PoC HCV screening and PoC liver fibrosis assessment.
PoC HCV screening was performed using the OraQuick® HCV Rapid Antibody test (OraSure Technologies, Bethlehem, PA, USA) via fingerstick with a drop of blood (100 mL).(10) The HCV serology result was obtained within 20 minutes.(11) PoC liver fibrosis assessment was performed using portable transient elastography (TE), FibroScan® (Echosens, Paris, France). All HCV-positive cases were advised to undergo PoC TE assessment, and randomly selected HCV-negative cases were invited to be screened to maintain confidentiality for the seropositive individuals. Those who refused HCV screening or survey questionnaires and those who had never used illicit drugs were excluded from the study.
There were several uncertain factors in this screening study, including the willingness of halfway house residents to participate, prevalence of HCV seropositivity, compliance to randomisation and linkage to care, and effectiveness of randomisation. As we were not certain regarding the effectiveness of direct access for linkage to care, we performed a feasibility study and allocated the HCV-positive cases into the two groups in a 2:1 ratio. Randomisation was performed by the central coordinator prior to the halfway house visit. In the first group, participants who were screened positive were given a referral letter to attend polyclinics with their results, with further instructions to refer them to CGH for specialist evaluation. This was the control group with standard access to care. In the intervention or direct access group, in addition to a direct referral letter, participants were given a hotline number to call to arrange for a direct clinic appointment at CGH, thereby circumventing the polyclinic referral by primary care physicians. Prior approval for this was obtained from the Ministry of Health, Singapore.
HCV-positive residents were linked to care at CGH, which is a 1,000-bed university-affiliated hospital located in the east of the island. The proportion of HCV-positive individuals who were linked to specialist care was determined by reviewing electronic medical records. Linkage of care cascade was defined as attendance at the specialist clinic, confirmation of viraemia by HCV RNA testing, discussion about HCV treatment and initiation of treatment. Intention-to-treat analysis was performed on the proportions of residents who were linked to care. At the time of the study, there was no universal DAA coverage, and the standard of care was pegylated interferon that required a 6–12-month treatment period under a government healthcare scheme. Owing to the long treatment course, the proportion of patients who were cured of HCV infection was not analysed.
The study participants were informed of the results of the HCV screening and TE assessment on the same day in person and in writing by on-site principal investigators (Thurairajah PH or Hsiang JC). The primary outcome measured was the proportion linked to each cascade of care, including rates of attendance at the specialist clinic, confirmation of viraemia by HCV RNA testing, discussion about HCV treatment with the specialist and initiation of treatment. All individuals were followed up for a period of four months from PoC testing to determine whether linkage of care had occurred.
Categorical data was presented as frequency. Continuous data was presented as mean ± standard deviation for parametric distributions and median ± interquartile range for non-parametric distributions. Differences in baseline characteristics between groups were examined using chi-square test or Fisher’s exact test for categorical variables, and two sample t-test and Mann-Whitney U test for continuous variables, where appropriate, with statistical significance defined as p-value < 0.05. Significant variables (p < 0.20) in univariate analysis that were associated with initiation of linkage to treatment were entered into a multivariate logistic regression model to determine clinical significance using odds ratio (OR) and 95% confidence intervals (CIs). Data was analysed using IBM SPSS Statistics version 23.0 (IBM Corp, Armonk, NY, USA).
RESULTS
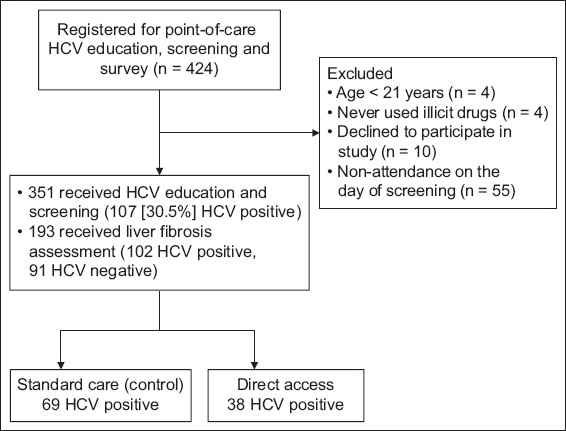
A total of 424 halfway house residents were pre-registered 1–2 weeks before HCV screening in 14 screening visits. A total of 73 residents were excluded for the following reasons: did not attend the screening (n = 55); under 21 years of age (n = 4); never used illicit drugs (n = 4); and refused to participate in HCV screening and survey on the day itself (n = 10). Hence, a total of 351 residents attended the HCV education seminar, completed the survey questionnaire and HCV screening, and were randomised into standard and direct access groups if they were screened as HCV positive (
Fig. 1
Flowchart shows the inclusion process of the study. HCV: hepatitis C virus
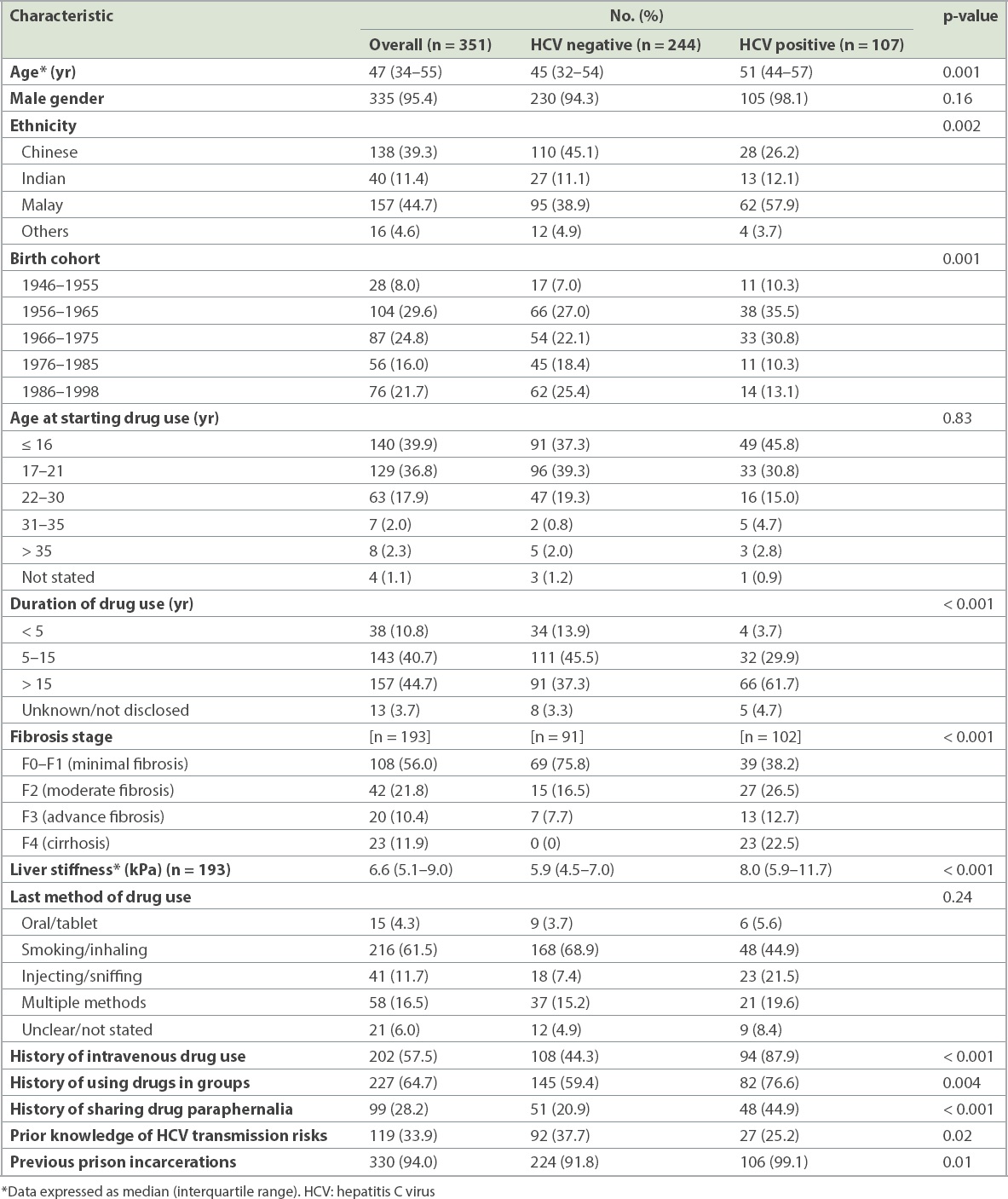
Of the 351 residents recruited, 95.4% were male. 39.3%, 44.7% and 11.4% were Chinese, Malay and Indian, respectively. All had a history of prior substance misuse and 202 (57.5%) of the 351 residents were previously PWID (
Table I
Baseline characteristics of halfway house residents by hepatitis C status.
A total of 69 patients in the standard care group and 38 in the direct access group were found to be HCV positive (
Table II
Baseline characteristics of HCV-positive cases for linkage to care.
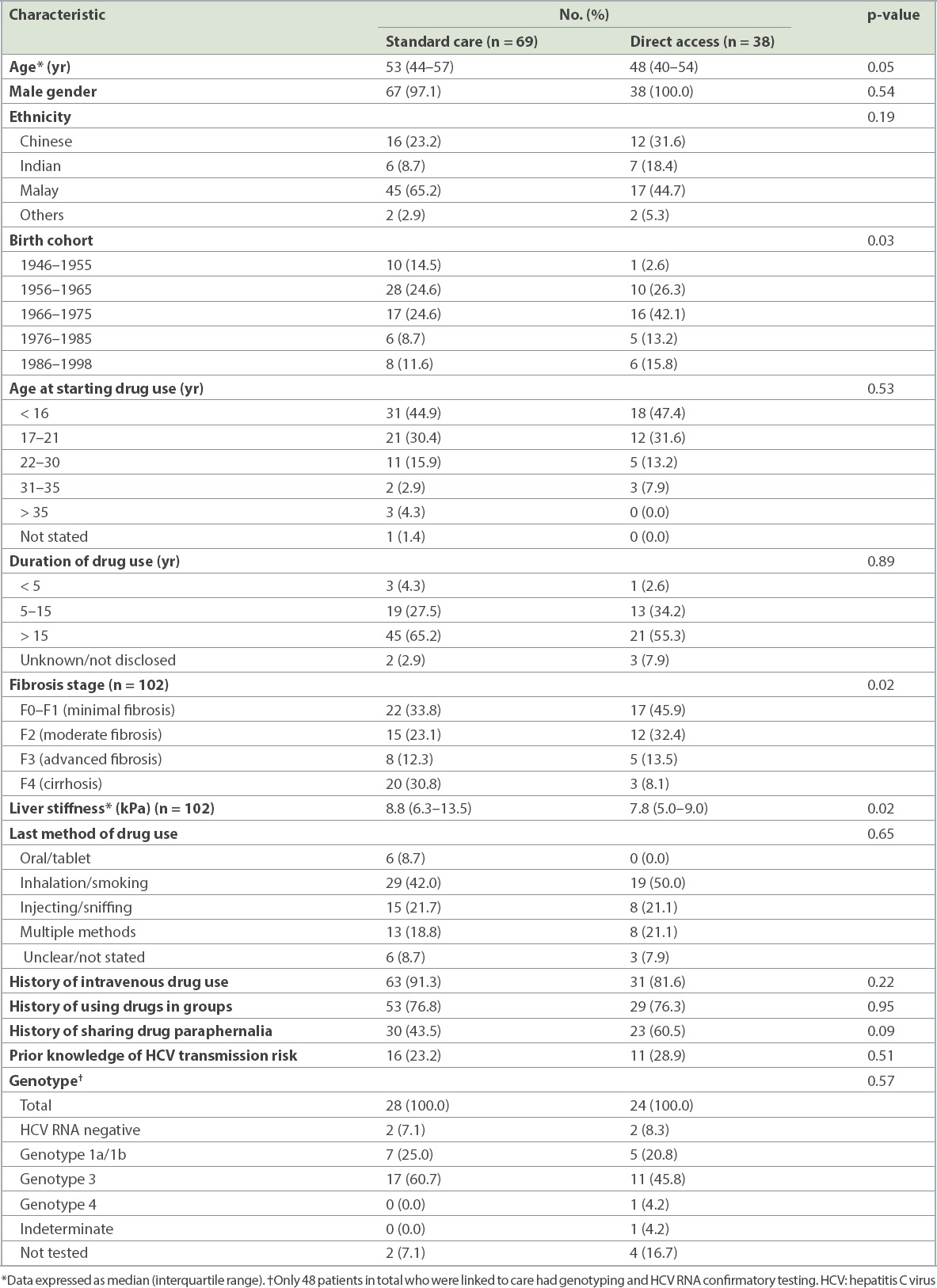
Fig. 2
Chart shows hepatitis C linkage-to-care cascade of halfway house residents.
Factors associated with linkage to treatment initiation from a decentralised PoC HCV screening programme with or without direct referral were analysed (
Table III
Factors associated with linkage to HCV treatment initiation.
DISCUSSION
In this prospective community study assessing the effectiveness of linkage to care models among an ex-substance misuse population in Singapore, we found that 30.5% of the individuals were HCV positive. The seroprevalence of HCV among PWID was 46.5%. Despite PoC HCV education and testing, the proportion of patients who were linked to specialist care was less than half of the cohort (48.6%), with only 26.2% of all patients ultimately having treatment discussions with specialists. Direct access improved the linkage to care compared to the usual referral pathway across the entire HCV care cascade. While the direct access group had a higher rate of initial referral to specialist compared to the control group, subsequently, a similar attrition of patients across the care cascade was observed as treatment discussion and initiation were approached. The rate of attrition within the two arms was similar, suggesting that other patient and treatment factors may be responsible for the high attrition rates. The study was conducted during the pegylated interferon era, when side effects to interferon may have been partially responsible for the poor treatment uptake and high dropout rates. Additionally, it is possible that some social and economic factors that remain a barrier to treatment uptake were not fully assessed by this study.(8) However, increasing the initial linkage to care by direct access improves the total number of patients who ultimately get treated, which is important for micro-elimination to be successful.
Within the present Singapore healthcare model, primary healthcare providers are required to make a referral to secondary care for treatment of HCV infection. This poses an additional barrier in terms of linkage to care owing to loss of income from time off work as well as the added financial burden of HCV antibody testing. This barrier impedes linkage to care within an underprivileged population, who typically have poor social and financial resources, may be unemployed and have poor engagement with healthcare services. POC testing and direct access circumvents many of these limitations and is considerably cheaper than laboratory serological testing.
Previous studies have shown the effectiveness of facilitated referral for high-risk populations,(12) PoC HCV screening,(13) education and TE assessment(14) in identifying the hidden HCV populations and linking them to care. To our knowledge, this is the first study to illustrate the effectiveness and feasibility of a combined approach of direct access referral following PoC HCV education, screening and TE assessment. This simplification of the care cascade may reduce cost to patients as well as the waiting period to see a specialist and staging with TE. Our study protocol may be applied to other urban populations to facilitate micro-elimination in key populations via PoC screening with subsequent specialist review in ‘walk-in’ clinics with expedited care for DAA treatment. In our experience, such a combined approach was neither difficult nor time-consuming. Ultimately, a universally subsidised DAA treatment programme empowering primary care to treat patients with HCV infection would minimise and simplify the care cascade issue among this high-risk population, with improvement in treatment coverage.(15) Indeed, Australia’s scale-up treatment coverage using the above strategy would allow it to meet WHO targets by the year 2028.(16)
In Singapore, HCV prevalence has been reported to be between 0.37% and 0.54% in the general population, based on blood donor prevalence studies.(17,18) The relatively low prevalence of HCV in the Singapore general population and high prevalence within halfway houses and correctional facilities make these hotspots ideal for HCV elimination. The HCV prevalence among the substance misuse population at halfway houses was high at 30.5%, which serves as a surrogate indicator of the overall prevalence of HCV in Singapore across all halfway houses and correctional facilities such as prisons. For elimination to be successfully implemented in an urban population such as that in Singapore, strategies with simplified access to diagnosis work-up and treatment that target this high-risk population at halfway houses and correctional facilities would yield the highest clinical effectiveness. Disrupting the chain of transmission among PWID is vital to reducing the burden of disease. Opioid substitution therapy (OST) used in conjunction with needle exchange programmes and in combination with universally accessible DAA has been shown to reduce the burden of HCV infection and is an effective adjunct in HCV elimination.(19)
This study has some limitations. First, less than 5% of the recruited subjects were women. This was because there is only one women’s halfway house in Singapore. Consequently, substance misuse among women is underrepresented within halfway houses. Women also make up a lower proportion of substance misuse cases in general. This was evident from our previous hospital-based studies on previous substance misuse with HCV,(20) where 7% of the population was female, suggesting that substance misuse is likely to be less prevalent among women in Singapore. Secondly, this was a pilot study with a small sample size. Nevertheless, it provides useful information on high-risk drug behaviour and important demographic data on HCV cases in halfway houses. Most importantly, this study provides early insight into an abbreviated HCV care cascade. Minimisation and abbreviation of the care cascade improves HCV treatment uptake. Further larger studies are warranted to show the cost-effectiveness of such community screening programmes, particularly POC treatment delivery, and the feasibility of this kind of HCV care models in countries with rural populations.
Thirdly, it is conceivable that some patients may have been referred to other hospitals, resulting in some loss of follow-up in the control arm. However, we do not anticipate that this would be a significant proportion of the cohort. Over 70% of the individuals screened were located in halfway houses near the east of Singapore. As this was within the CGH catchment area, the polyclinics would have referred patients to us. While carrying out the HCV education and screening, we established a rapport with the subjects, which encouraged the positive patients to attend follow-up consultations in CGH. Finally, we were unable to complement the simplified care cascade with universal DAA treatment. It would have allowed us to assess cure rates and outcomes from linkage to treatment. During the study period, a government-negotiated DAA price scheme at reduced cost had not come into effect, and therefore, some patients received DAA based on the severity of their liver disease rather than patient or clinician preference. Linkage to care for people with substance misuse may greatly improve once the cost of DAA treatment becomes both affordable and universally accessible.
In conclusion, this study showed that decentralised PoC education and screening within a population at high risk for HCV are effective tools for case identification. PoC liver fibrosis assessment revealed a high proportion of cirrhosis within this study cohort, suggesting that the disease burden could rise further over the next ten years unless HCV elimination strategies are instituted. Linkage to care remains poor among PWID populations despite the PoC HCV care model and facilitated referrals. This is likely owing to multiple social and financial reasons, and simplification of the HCV care cascade would improve treatment uptake. PoC HCV screening, diagnosis and treatment may hence be an effective strategy towards achieving HCV elimination in Singapore. For an effective public health strategy aiming to eliminate HCV, further studies are needed to determine the number of PWID required to treat per annum to attain the WHO’s 2030 goal of reducing HCV incidence and liver-related mortality in Singapore.
ACKNOWLEDGEMENTS
This study received an investigator-initiated research grant from Gilead Sciences and a National Medical Research Council centre grant. We would like to thank OraSure Technologies for its support in this study and the Ministry of Health, Singapore, for its support and assistance with the ‘direct access’ referrals. We would like to acknowledge Ms Samantha Wee and Ms Nur Shameerah Binte Abdul Halim, who helped with the coordination of study visits. We would like to thank Dr Eugene Wong, Dr Marianne De Roza and Dr Jessica Tan for their voluntary assistance with screening. Finally, we would like to thank all the residents who participated in this study and the halfway house personnel for their support and assistance.


