Abstract
INTRODUCTION
The prognosis of patients with chronic myeloid leukaemia (CML) has improved since the introduction of imatinib. However, patients who do not achieve complete cytogenetic response (CCyR) and major molecular response (MMR) have poorer prognosis. Recent clinical trials have demonstrated that early and deeper cytogenetic and molecular responses predict a better long-term outcome. This study aimed to analyse the relationship between early molecular response and clinical outcome in a real-life setting.
METHODS
This retrospective study included all patients with CML, in chronic or accelerated phase, who were treated with imatinib at University of Malaya Medical Centre, Malaysia.
RESULTS
A total of 70 patients were analysed. The median follow-up duration was 74 months, and the cumulative percentages of patients with CCyR and MMR were 80.0% and 65.7%, respectively. Overall survival (OS) and event-free survival (EFS) at ten years were 94.3% and 92.9%, respectively. Patients who achieved CCyR and MMR had significantly better OS and EFS than those who did not. At six months, patients who had a BCR-ABL level ≤ 10% had significantly better OS and EFS than those who had a BCR-ABL level > 10%. The target milestone of CCyR at 12 months and MMR at 18 months showed no survival advantage in our patients.
CONCLUSION
Our data showed that imatinib is still useful as first-line therapy. However, vigilant monitoring of patients who have a BCR-ABL level > 10% at six months of treatment should be implemented so that prompt action can be taken to provide the best outcome for these patients.
INTRODUCTION
Chronic myeloid leukaemia (CML) is now considered an indolent disease. This was achieved after imatinib mesylate, a selective BCR-ABL tyrosine kinase inhibitor (TKI), was introduced for the treatment of CML a decade ago. In fact, 90% of CML patients on imatinib mesylate treatment are still alive after five years.(1) Furthermore, patients who achieve complete cytogenetic response (CCyR) and major molecular response (MMR) by 12 and 18 months, respectively, show significantly better progression-free survival (PFS).(2) Nevertheless, there is still a substantial number of patients who do not achieve the desired response. An eight-year follow-up conducted in the Immediate Risk Stratification Improves Survival (IRIS) trial showed that 37% of the patients on imatinib had an unfavourable outcome, with 32% of them failing to achieve CCyR or losing CCyR, and 5% of them being intolerant to imatinib.(3)
Many studies have demonstrated that the achievement of early CCyR and MMR among imatinib-treated CML patients is associated with improved survival.(4-7) Based on the findings of these studies, molecular response at defined time points is now widely accepted as a predictive marker for subsequent outcomes in CML patients. Second-generation TKIs, such as dasatinib, nilotinib and bosutinib, are more potent than imatinib, and have proven efficacy in patients who are resistant or intolerant to imatinib.(8,9) More patients have achieved optimal cytogenetic and molecular responses when treated with these newer drugs than with imatinib. Furthermore, the responses achieved using the newer drugs were faster and deeper, contributing to a lower rate of disease progression and superior long-term outcomes.(10-12)
Both the United States’ National Comprehensive Cancer Network (NCCN) and the European LeukemiaNet (ELN) updated their guidelines to include BCR-ABL ≤ 10% as a treatment response milestone at three months.(13,14) ELN defines optimal response as a BCR-ABL level < 1% at six months and ≤ 0.1% at 12 months, while NCCN defines it as CCyR, with or without MMR, by 12–18 months. In addition, a BCR-ABL level > 10% at six months and > 1% at 12 months is defined by ELN as treatment failure that requires a switch of TKIs. On the other hand, NCCN recommends a change to alternate TKIs if the BCR-ABL levels are > 10% at three months or if Philadelphia (Ph) chromosome-positive cells are present at 12 months.
As Malaysia is a developing country without comprehensive medical insurance coverage, most CML patients in Malaysia are dependent on free imatinib provided through the Malaysia Patient Assistance Programme. It is extremely difficult for the treating haematologist to adjust the treatment protocol according to the international guidelines, as the majority of patients are unable to afford the cost of newer TKIs. Therefore, the objectives of the present study were to: (a) analyse the outcome of treatment with imatinib; and (b) confirm if early achievement of molecular responses according to the ELN and NCCN guidelines predicted better survival in CML patients who were treated with imatinib. The findings of this study would help to shed light on the outcomes of CML patients who are not treated according to the ideal protocol (i.e. the guidelines recommended by ELN and NCCN).
METHODS
This observational, retrospective study included all patients who were diagnosed with chronic- or accelerated-phase CML and treated with imatinib at University of Malaya Medical Centre, Kuala Lumpur, Malaysia. The patients’ characteristics, molecular and cytogenetic responses to imatinib, and survival probabilities were examined.
The patients’ cytogenetic response was based on the prevalence of Ph chromosome-positive metaphases among at least 20 metaphases investigated in each bone marrow sample. Cytogenetic response was defined as: (a) complete, if there were 0% Ph chromosome-positive cells in metaphase; (b) partial, if 1%–35% were Ph chromosome-positive cells in metaphase; and (c) non-existent, if > 35% were Ph chromosome-positive cells in metaphase. Both complete and partial cytogenetic responses (i.e. Ph chromosome-positive cells = 0%–35%) were considered as major cytogenetic responses in this study. Molecular monitoring was done by measuring the number of BCR-ABL transcripts in peripheral blood. This was done using reverse transcription-polymerase chain reaction (RT-PCR) in our laboratory, which is compliant with international standards. MMR was defined as a BCR-ABL/ABL ratio ≤ 0.1%, while complete molecular response referred to the absence of detectable BCR-ABL transcripts via RT-PCR, at a sensitivity > 10–4.5.
The endpoints we examined were death from any cause during treatment, disease progression into more advanced phases, and loss of complete haematologic and/or major cytogenetic responses. The duration of response was calculated from the first reported date of response to the earliest date of reported relapse or death. Time to progression was defined as the time from the start of the treatment to the onset of: (a) an accelerated or a blastic phase; (b) discontinuation of therapy due to unsatisfactory therapeutic effects; or (c) death. Survival was calculated from the beginning of therapy until the time of death from any cause.
SPSS for Windows version 16.0 (SPSS Inc, Chicago, IL, USA) was used to process, manage and analyse the data to determine the factors that significantly affect the response rate, survival and frequency of adverse effects. Frequencies and descriptive statistics were also generated using the same program. Differences among the variables were evaluated using chi-square test, and survival probabilities were estimated using Kaplan-Meier’s method. A p-value < 0.05 was considered statistically significant.
RESULTS
A total of 70 patients were included in the study – 65 (92.9%) in chronic phase and 5 (7.1%) in accelerated phase (
Table I
Demographics of the patients (n = 70).
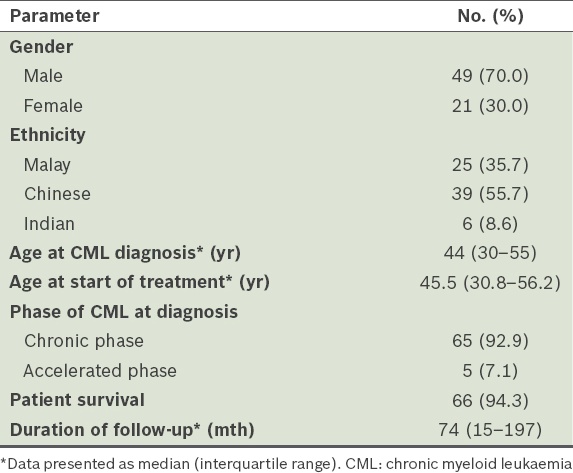
The overall survival (OS) at ten years was 94.3% (
Fig. 1
Graph shows the overall duration of survival of the patients (n = 70).
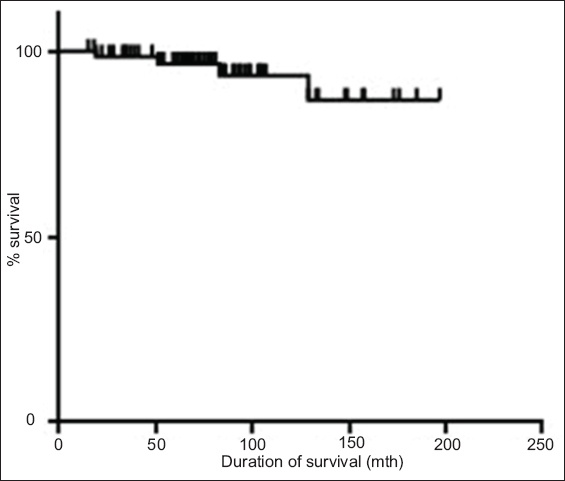
Fig. 2
Graph shows the duration of survival of the patients according to whether they achieved complete cytogenetic response (CCyR).
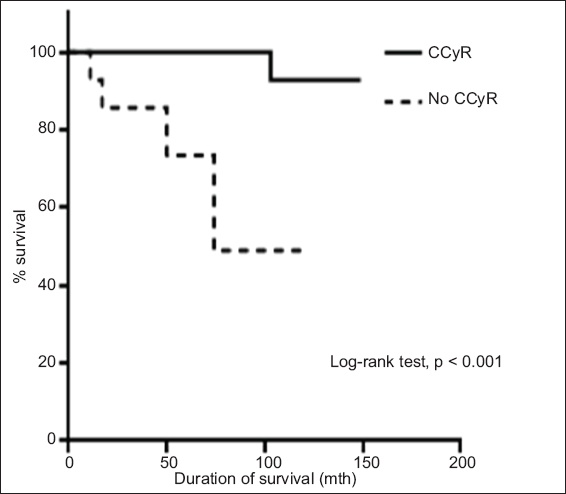
Table II
Outcomes following treatment with imatinib.
Fig. 3
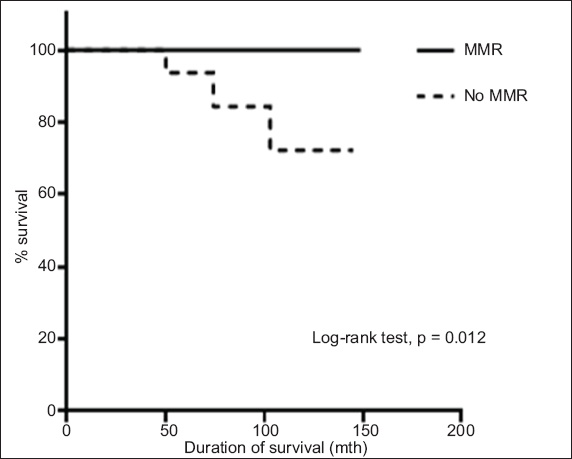
Graph shows the duration of survival of the patients according to whether they achieved major molecular response (MMR).
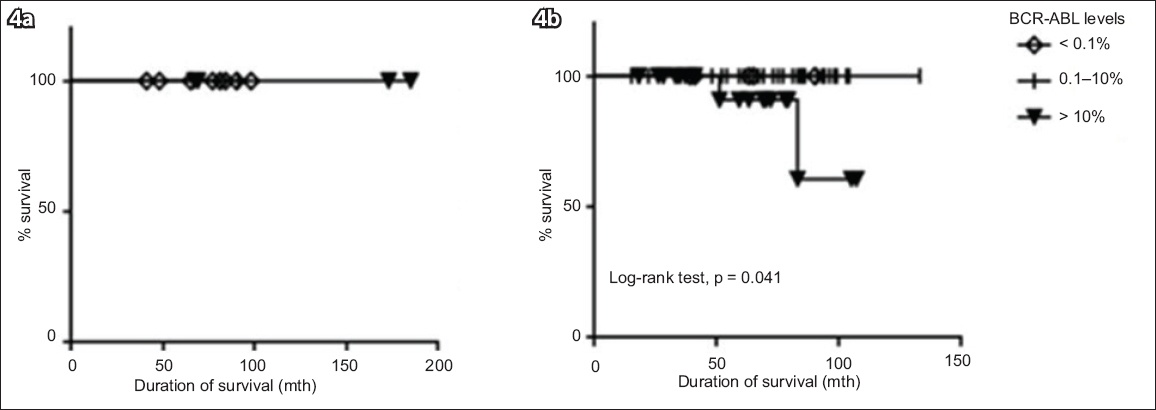
Patients who had a BCR-ABL level ≤ 10% at six months had significantly better OS than those who had a BCR-ABL level > 10% at six months (100.0% vs. 88.9%, respectively; p = 0.041) (
Fig. 4
Graphs show the duration of survival of the patients, according to their BCR-ABL levels at (a) three months and (b) six months.
The ten-year event-free survival (EFS) among the 70 patients was 92.9%. The EFS was better among the patients who achieved CCyR and MMR than those who did not (CCyR: 96.4% vs. 73.0%, respectively; p = 0.002 and MMR: 97.8% vs. 92.9%, respectively; p = 0.003). The EFS was also better among the patients who had a BCR-ABL level ≤ 10% at six months than those who had a BCR-ABL level > 10% at six months (100.0% vs. 82.4%, respectively; p = 0.013). There was no significant difference in the EFS of the patients who achieved MMR at or before 18 months and the EFS of those who did not (100.0% vs. 96.2%, respectively; p = 0.366). Similarly, there was no significant difference in the EFS of the patients who achieved CCyR at or before 12 months and those who did not (100.0% vs. 91.7%, respectively; p = 0.319). On the other hand, we found that a < 1 log reduction of BCR-ABL at 12 months predicts poorer outcome; the EFS was 81.8% and 100.0% for patients who had < 1 log reduction of BCR-ABL and those who had ≥ 1 log reduction of BCR-ABL at 12 months, respectively (p = 0.006).
DISCUSSION
The survival of patients with CML has improved significantly since the introduction of imatinib more than a decade ago. Early-phase CML patients receiving imatinib can have an almost-normal lifespan. The IRIS trial showed that the eight-year survival rate for CML patients on imatinib was 85%; the survival rate was even better (i.e. 93%) if only CML-related deaths were considered.(2,3) In the present study, the patients on imatinib showed a similarly excellent survival rate, with a median survival duration of 74 (range 15–197) months and OS of 94.3%. In the IRIS trial, long-term follow-up of the patients also revealed that nearly all the patients who remained on imatinib achieved CCyR, and progression of the disease after the first three years of treatment was extremely rare.(2) In comparison, the percentage of CCyR and MMR among the patients in the present study was 80.0% and 65.7%, respectively, which are comparable to other studies.(15,16) Hence, the results of our study show that a good response can also be achieved in a real-life clinical setting.
Even though imatinib has been proven to have excellent efficacy, there are still a considerable number of patients who respond poorly to it. Therefore, many studies have been conducted to further improve the outcome of CML patients. Hehlmann et al compared imatinib 800 mg/day, imatinib 400 mg/day and imatinib 400 mg/day plus interferon to determine if more intensive treatment would result in a better outcome.(17) The results of their study showed that patients on high-dose imatinib (i.e. 800 mg/day) had a significantly higher incidence of MMR at 12 months. However, the better responses did not translate to better OS and PFS at three years.(17) This observation was further confirmed in a systematic review and meta-analysis by Gafter-Gvili et al.(18) In that review of four randomised trials, the authors showed that CCyR and MMR at 12 months were improved by high-dose imatinib in patients with chronic-phase CML; however, there was no difference in all-cause mortality or disease progression at the end of follow-up.(18) The results of the present study revealed that the patients who achieved CCyR and MMR had better OS and EFS as compared to those who failed to achieve these responses (
In a retrospective analysis of 1,440 newly diagnosed chronic-phase CML patients, OS rates at five years were better among the patients who had a BCR-ABL level between 1% and 10% at three months (OS 94%) and ≤ 1% (OS 97%) at three months, as compared to the patients who had a BCR-ABL level > 10% at three months (OS 87%).(19) In addition, a BCR-ABL level > 1% at six months was associated with inferior survival rates at five years when compared to a BCR-ABL level < 1% at six months (89% vs. 97%).(19) Jain et al published similar evidence from their single-centre experience with 483 newly diagnosed chronic-phase CML patients who received either 400 mg or 800 mg of imatinib, nilotinib or dasatinib as first-line treatment.(20) The three-year failure-free survival (FFS) was better if the three-month BCR-ABL level was ≤ 1% and not between 1% and 10% or > 10% (FFS 85%, 73% and 61%, respectively; p = 0.016). The three-year FFS for the patients with a six-month BCR-ABL level ≤ 1% was 89%, while it was 56% for patients with a BCR-ABL level between 1% and 10%, and 49% for those with a BCR-ABL level > 10% (p < 0.001).(20)
The ENESTnd (Evaluating Nilotinib Efficacy and Safety in clinical Trials-newly diagnosed patients) study, which compared the use of nilotinib and imatinib in newly diagnosed chronic-phase CML patients, also reported correlations between BCR-ABL levels at three months, and PFS and OS by four years.(9) Similar results were seen in the DASISION (DASatinib versus Imatinib Study In treatment-Naive CML patients) trial, which compared the use of dasatinib and imatinib in newly diagnosed chronic-phase CML patients.(8) At the end of three years, better PFS and OS were observed in the patients with a three-month BCR-ABL level ≤ 10% than in the patients with a three-month BCR-ABL level > 10% (PFS: 93% vs. 68%, p = 0.0003; OS: 96% vs. 86%, p = 0.03).(8) Marin et al identified that patients with a BCR-ABL level < 9.84% at three months and < 1.67% at six months had significantly higher eight-year OS than patients with higher transcript levels (93.3% vs. 56.9% and 93.7% vs. 74.7%, respectively).(21) In contrast, a BCR-ABL level > 10% at six months predicted poorer survival in the patients in the present study; at six months, the OS for patients with a BCR-ABL level ≤ 10% and patients with a BCR-ABL level > 10% were 100.0% and 88.9%, respectively (p = 0.041). This finding is consistent with the results published by Klamová et al, which showed that patients with a BCR-ABL level < 10% at six months had better survival.(16) Furthermore, Hughes et al also proved that BCR-ABL levels of > 10% at six months and > 1% at 12 months predict inferior EFS and a higher rate of disease progression.(2) This observation is especially important for less developed countries, where the need to use higher dosages of imatinib or the need to change treatment from imatinib to newer-generation TKIs (in order to achieve faster and deeper responses) would result in a significant financial burden.
The present study also revealed that at 12 months, ≥ 1 log reduction of BCR-ABL predicts better EFS when compared to < 1 log reduction of BCR-ABL (100.0% vs. 81.8%, respectively; p = 0.006), even though no significant difference was observed between the OS of these two groups (100% vs. 91.3%, p = 0.142).
Second-generation TKIs are more potent, and induce faster and higher rates of CCyR and molecular responses. They have also recently been approved as first-line therapy. While there is a trend of better PFS with these newer TKIs, there is no evidence to show improved OS.(22) Imatinib is still advantageous since it is effective in a large proportion of patients. In addition, its safety profile is well established. More importantly, the imminent availability of generic imatinib could potentially reduce the financial burden of long-term therapy for CML. This is crucial, especially in developing countries, as experts have argued that the prices of cancer treatment, with CML treatment as the example, are not sustainable.(23)
In conclusion, the results of the present study show that imatinib is still useful as first-line therapy. However, vigilant monitoring of patients whose BCR-ABL level is > 10% at six months of treatment should be implemented so that prompt action can be taken to provide the best outcome for these patients.


