Abstract
INTRODUCTION
Emesis is one of the most common adverse events associated with ketamine sedation. However, its predictors have not been clearly studied among Asian children. This study aimed to determine the incidence and predictors of emesis in children undergoing intramuscular (IM) ketamine sedation in an emergency department (ED) in Singapore and to identify high-risk groups, so that antiemetics may be administered prophylactically.
METHODS
In a prospective observational study, all children requiring procedural sedation with IM ketamine in the paediatric ED between 1 April 2013 and 31 January 2015 were included. All cases of emesis following ketamine sedation were prospectively documented. Univariate and multivariate logistic regression analyses were performed to identify the predictors of emesis.
RESULTS
2,502 sedations were performed using IM ketamine in the ED during the study period. Overall incidence of emesis associated with IM ketamine sedation was 8.4%. Children aged ≥ 8 years were significantly associated with increased risk of emesis (odds ratio 4.636, 95% confidence interval 3.271–6.570; p < 0.001), with an emesis rate of 19.6%. Other variables such as initial dose (3 mg/kg vs. 4 mg/kg), total dosage (including top-ups), type and site of procedure, gender and ethnicity were not significant predictors. The number needed to treat for antiemetics in children aged ≥ 8 years was five.
CONCLUSION
Age is a significant predictor of emesis. We recommend conducting a randomised controlled trial to compare the effects of prophylactic oral ondansetron in patients stratified into the age groups of ≥ 8 years and < 8 years.
INTRODUCTION
Over the last 20 years, ketamine has become an essential drug in the paediatric emergency department (ED).(1,2) Being a dissociative agent, it is used as a procedural sedation and analgesic agent for children.(3) This dissociation results in a trance-like cataleptic state that is characterised by an effective combination of analgesia, sedation and amnesia.(4) Ketamine is also easily administered either intramuscularly or intravenously.(5,6) These properties, along with its low risk profile, make ketamine a popular drug in the ED.(7-9)
Certain adverse events have been associated with the use of ketamine. These include airway events, such as laryngospasm and apnoea, hypersalivation, emesis and recovery agitation.(10-13) Among them, ketamine-associated vomiting is the most common adverse event.(13,14) Although not a serious outcome, it is common enough to result in delayed discharge and leads to poor parental satisfaction with sedation. The rate of ketamine-associated emesis in paediatric patients has been reported to be in the range of 6%–28%, depending on the size of the study.(14-17) Although a meta-analysis of 8,202 children showed that age and higher dosage of ketamine were associated with increased vomiting, no known published study has been done on Asian children.(12)
The objectives of this study were to determine the incidence and predictors of emesis in local children undergoing intramuscular (IM) ketamine sedation in the ED of a paediatric tertiary hospital, and to identify high-risk groups, so that antiemetics may be administered prophylactically.
METHODS
This was a prospective observational study conducted on paediatric patients who presented to the ED at KK Women’s and Children’s Hospital, Singapore, between 1 April 2013 and 31 January 2015. The study protocol was approved by the hospital’s institutional review board. All patients who underwent procedural sedation with IM ketamine were included. Children who received intravenous (IV) ketamine or another primary sedative, such as fentanyl or nitrous oxide, were excluded from the study.
All patients underwent procedural sedation in the ED treatment room, which was fully equipped for monitoring and resuscitation. Fasting for solids for three hours prior to sedation was ensured for all patients, according to department clinical guidelines. An adverse event form was used to record all intraprocedural events and any adverse events, such as emesis, after ketamine sedation. The discharge criteria included patent airway with normal oxygenation and return to pretreatment levels of awareness and verbalisation.(2,18)
The basic demographic information of all patients undergoing IM ketamine sedation was extracted from the electronic database of the ED. For patients who experienced emesis post sedation, their respective adverse event forms were examined to extract relevant data regarding the number of times emesis occurred, any delayed discharge, relevant interventions administered (such as oral or IV ondansetron) and the need for IV hydration. All of the above data was then exported into Microsoft Excel for further analysis.
The primary outcomes were the rate of emesis post IM ketamine sedation and the predictor(s) of emesis. The predictors analysed included patient age, type of procedure for which the patient was undergoing sedation, site of procedure (head and neck, or rest of the body), initial dose of IM ketamine (3 mg/kg or 4 mg/kg), any additional dose of ketamine or other additional sedative, such as IV midazolam administered after the initial IM ketamine (i.e. top-ups), and time of day when the procedure was performed. Basic descriptive statistics were used to summarise the dataset. To further define the effect of age as a predictor of emesis, a frequency distribution for age was plotted against the corresponding rate of emesis. In order to better characterise its relationship with emesis, age was dichotomised into a categorical variable, with children grouped into two different categories – aged 0–7.9 years and aged 8–16 years. This variable was then used to run further analyses using univariate and multivariate logistic regressions.
All categorical variables were analysed using chi-square test, while independent t-test was used for continuous variables. Following this, univariate and multivariate regression analyses were performed, with emesis as the primary outcome.(19) The odds ratio (OR) for each predictor was calculated from the model. Finally, Hosmer-Lemeshow test was performed for a goodness of fit analysis. All statistical analyses were performed using IBM SPSS Statistics version 21.0 (IBM Corp, Armonk, NY, USA).
RESULTS
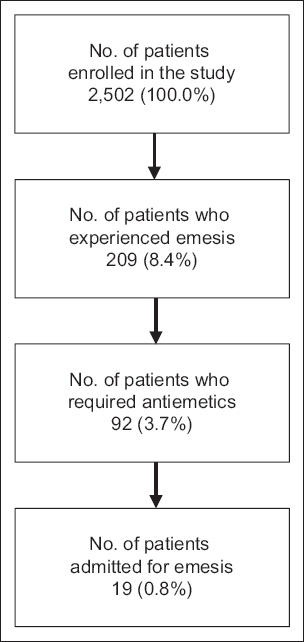
During the study period of 22 months, a total of 2,502 patients who received IM ketamine for procedural sedation were enrolled in the study. Basic demographic information on gender and ethnicity were available for 1,320 and 1,329 children, respectively. Among these children, emesis was documented in 209 (8.4%) patients. 92 (3.7%) children required administration of an antiemetic (ondansetron) as treatment for vomiting. 19 (0.8%) children had to be admitted for IV rehydration due to persistent symptoms after administration of antiemetics (
Fig. 1
Flowchart shows the inclusion process for the present study.
Baseline characteristics of children with emesis associated with IM ketamine sedation are shown in
Table I
Baseline characteristics of children with emesis associated with IM ketamine sedation.
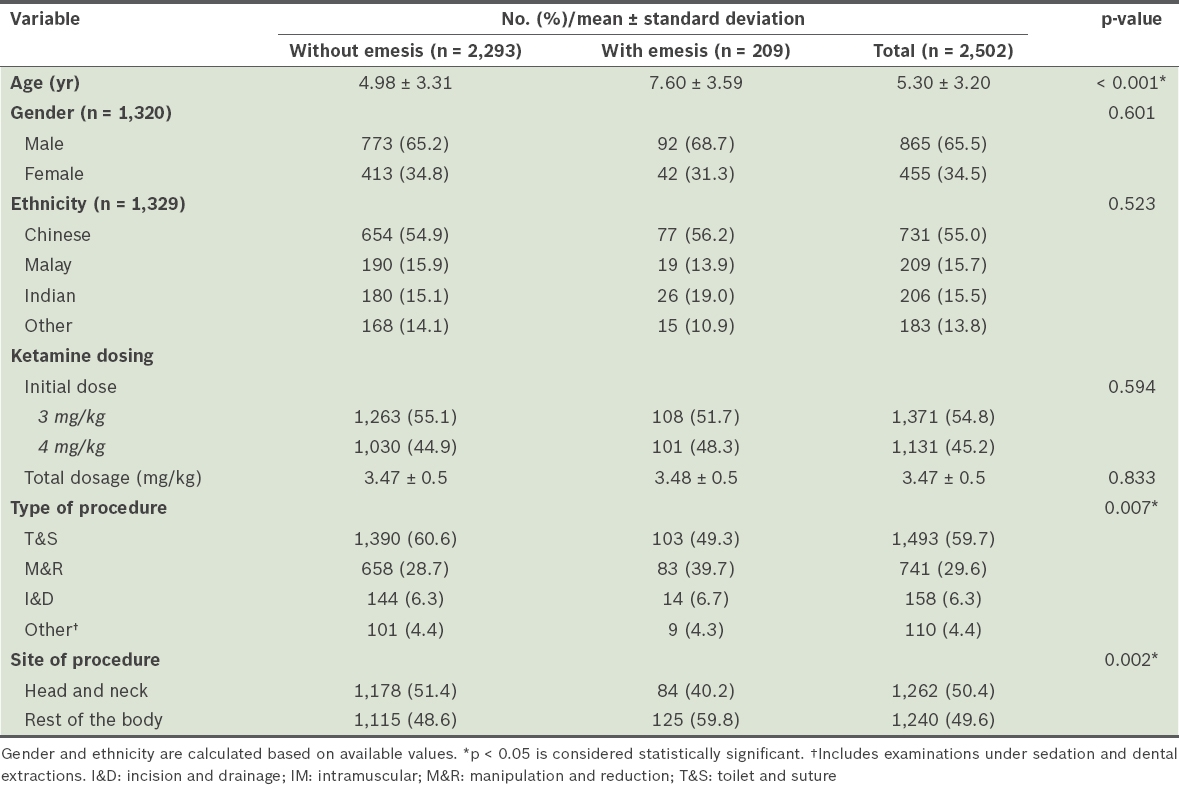
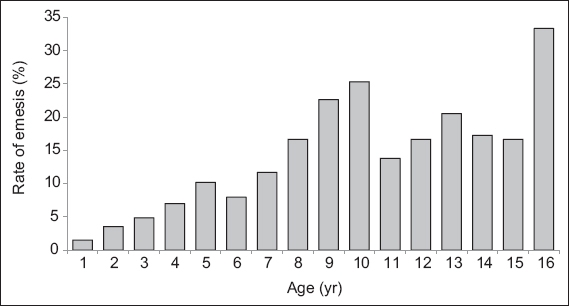
Fig. 2
Bar chart shows the frequency distribution of emesis stratified by age of the children.
The emesis rate was 19.6% for children aged ≥ 8 years as compared to 5.5% for children aged < 8 years (
Table II
Rate of emesis and emesis-related complications, stratified by age.
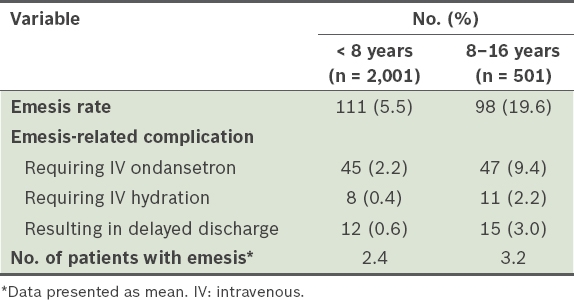
Table III
Summary of univariate and multivariable logistic regression analyses.
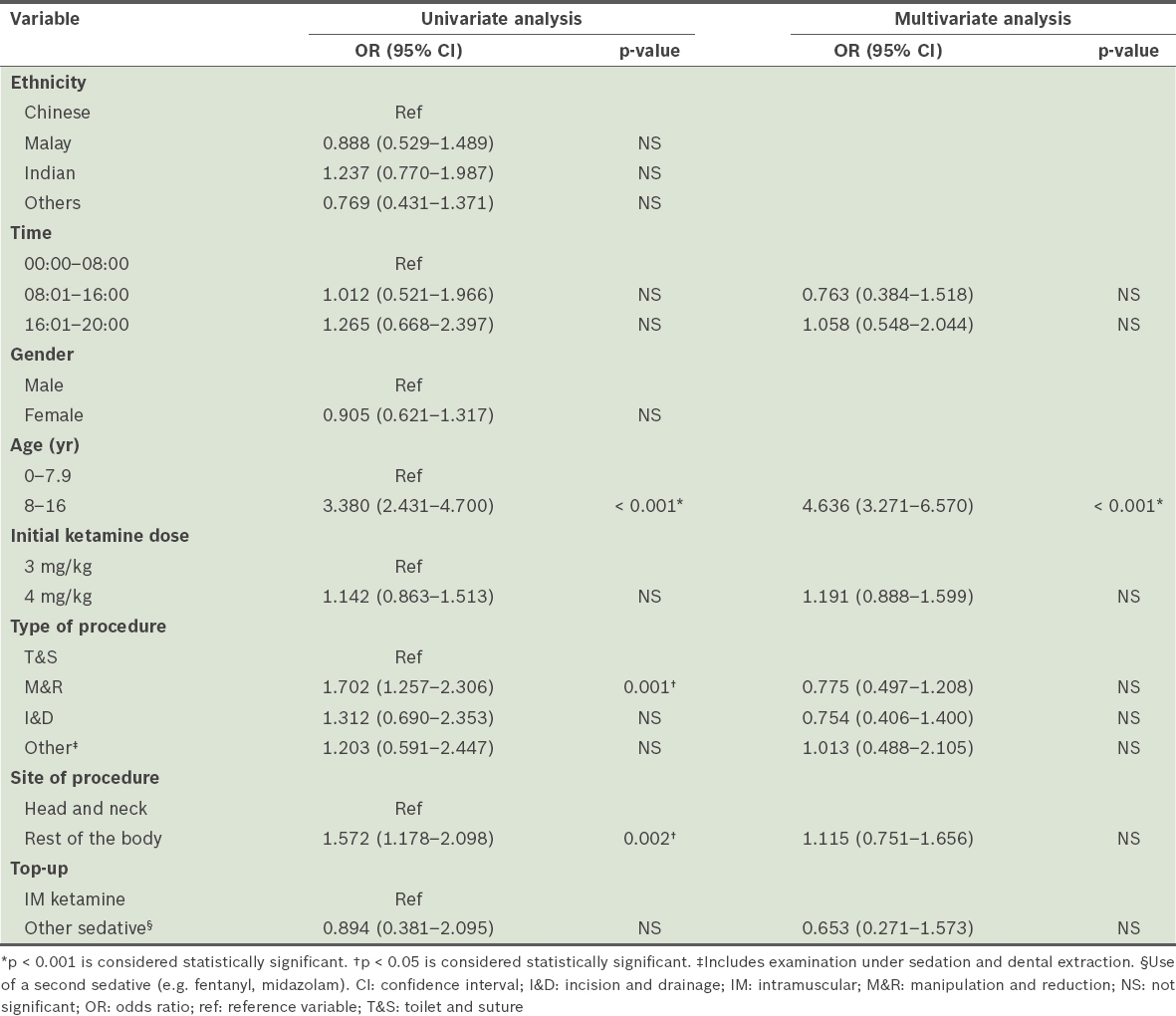
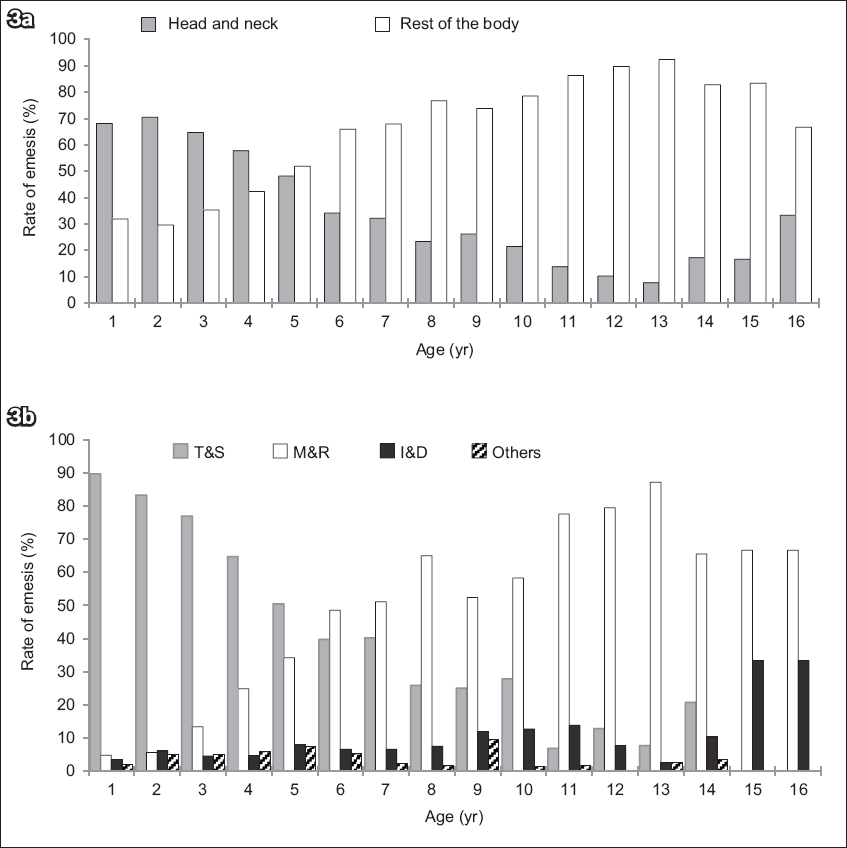
Though the type and site of procedure were significant predictors of emesis on univariate analysis, the only independent predictor of emesis that remained significant after multivariate analysis was age ≥ 8 years. This was likely due to fewer procedures involving the head and neck region and the increasing number of manipulation and reduction (M&R) procedures being performed in this group of children (
Fig. 3
Bar charts show the proportion of procedures stratified by (a) age and site of procedure and (b) age and type of procedure. I&D: incision and drainage; M&R: manipulation and reduction; T&S: toilet and suture
DISCUSSION
To the best of our knowledge, this is the largest single-centre report of emesis following IM ketamine sedation in the paediatric ED, presenting data for 2,502 patients over a period of 22 months in Singapore.(14) This was also the first study that aimed to identify the risk factors of emesis in children undergoing IM ketamine sedation in our diverse local population. Previous studies have reported that the rate of emesis following ketamine sedation in paediatric patients varied widely, ranging between 6% and 28%.(14-17) However, some of these studies included any emesis that occurred up to 24 hours after discharge from hospital. In the present study, emesis was defined as having at least one episode of vomiting occurring before discharge from the ED. Episodes of vomiting after discharge were not considered in our analysis.
Age was a significant predictor in all stages of statistical analysis in this study. This is in agreement with previous studies that concluded that the rate of emesis reached a maximum level at the age of 12 years.(12,21,22) While we concur that patient age plays an important role in the rate of adverse events, our analysis showed that all children aged ≥ 8 years were at a higher risk of vomiting following IM ketamine sedation.
As children aged ≥ 8 years have a higher risk of emesis, administering prophylactic antiemetics (such as ondansetron) for these patients, as proposed in some studies,(12) may reduce the risk of emesis associated with ketamine sedation. Using the incidence of emesis (19.6%) for children aged > 8 years, the number needed to treat for antiemetics would be at least five to prevent one incidence of emesis in this age group. This compares with the findings of an earlier meta-analysis, which reported that nine was the number needed to treat.(12)
Univariate logistic regression analysis showed that children undergoing a procedure in a site other than the head and neck region were more likely to experience post-sedation emesis than those with procedures in the head and neck region. The same was also seen in children undergoing M&R procedures during ketamine sedation. However, these factors were no longer significant on multivariate logistic regression analysis. We postulate that one possible explanation for this discrepancy is that a majority of children who undergo M&R procedures under ketamine sedation are generally older than those undergoing other procedures, such as toilet and suture. All M&R procedures are performed on the upper and lower limbs of the patient’s body, which means that a majority of patients who had procedures done in the rest of the body were older than those who had procedures in the head and neck region. This relationship between patient age and the type and site of procedure is evident in
Although some studies have suggested the need for a lower dose of ketamine to minimise the risk of adverse events, a meta-analysis of 8,282 children showed that after excluding high IV doses, there was no relationship between dose and adverse events.(23,24) Our findings were similar to these studies, in that the initial dose of IM ketamine administered (3 kg/mg or 4 kg/mg) did not predispose the patient to emesis.
Ondansetron has been widely used in the clinical setting to prevent and treat vomiting in children with various conditions ranging from viral illness to chemotherapy.(25-27) It has also been useful in the setting of anaesthesia.(17) However, its use in preventing emesis following ketamine sedation has seen mixed views.(17,21) For instance, a prospective study in 2014 showed no improvement in the rate of emesis with the administration of prophylactic oral ondansetron for IM ketamine sedation.(21) However, the mean age of the children who participated in the study was 30 months, which is much lower than the age range of the at-risk adolescent population. Another study, which looked at the administration of IV ondansetron with IV ketamine, showed a marked improvement in the emesis rate.(17) The mean age of patients in that study was 7.8 years, further supporting the hypothesis that age is an important risk factor. However, both of the aforementioned studies on the effectiveness of prophylactic ondansetron failed to perform risk stratification in terms of age, or to compare the findings between younger and older children. As the role and effectiveness of ondansetron for preventing emesis post IM ketamine sedation is not clearly demonstrated in the current literature, a randomised controlled trial that compares the effectiveness of oral ondansetron between stratified groups of younger versus older children is warranted.
The present study was novel in that it only looked at the administration of IM ketamine as a cause for emesis post sedation; the IV route, which is also a common mode of delivery for ketamine, was not analysed here. In contrast to previous studies that were performed to identify risk factors for emesis, the patient group consisted of patients who were administered either IV or IM ketamine.(12,15) Thus, unlike these earlier studies, the risk factors identified from our study were specific to the use of IM ketamine for procedural sedation.
A limitation of the present study was that delayed emesis that occurred after discharge from the hospital was not included in the analysis due to lack of data. This could have limited the number of adverse events recorded, as studies have suggested that up to 18% of children are known to have emesis during the first 24 hours post sedation.(28) Furthermore, we did not consider the patient’s body mass index in the analysis, and studies have reported that children with a higher body mass index are more prone to emesis after IV ketamine sedation.(29) It would be interesting to examine whether body mass index has a similar association with IM ketamine.
In summary, we found that the overall risk of ketamine sedation-associated emesis in paediatric patients was 8.4% and children aged ≥ 8 years were at a higher risk of emesis following ketamine sedation (19.6%). This data could be useful to clinicians when counselling parents of children who are scheduled to undergo procedural sedation using IM ketamine. We suggest that a randomised controlled trial be performed to study the benefit of the administration of prophylactic oral ondansetron for ketamine sedation in children, stratifying patients based on age (under and over eight years).
ACKNOWLEDGEMENT
We would like to express our appreciation to Ms Nivedita Vikas, Assistant Professor, Centre of Quantitative Medicine, Duke-NUS Medical School, Singapore, for her oversight of the statistical analyses.


