Abstract
INTRODUCTION
This study aimed to identify predictors of the outcome and clinical efficacy of emergency pelvic artery embolisation (PAE) for primary postpartum haemorrhage (PPH) and to assess the post-embolisation fertility of PAE patients in a regional hospital setting.
METHODS
A 12-year retrospective study of patients undergoing emergency PAE was conducted at a regional acute general hospital. Clinical and procedural parameters, clinical outcomes and post-embolisation pregnancy success rates were analysed.
RESULTS
There were 47,221 deliveries at the hospital during the study period, of which 33 patients required urgent PAE for primary PPH. The technical success rate of embolisation was 97.0% (n = 32). Clinically adequate haemostasis was achieved by a single embolisation procedure in 24 (72.7%) patients; the remaining eight eventually required surgery to achieve cessation of bleeding. Among the parameters studied, multivariate logistic regression analysis showed that pre-embolisation platelet count (p = 0.036) and maternal age (p = 0.019) were the only significant independent predictors of embolisation failure. Only two patients successfully conceived after PAE, although one of them had an ectopic pregnancy.
CONCLUSION
Emergency PAE is an effective measure to arrest life-threatening bleeding in patients with primary PPH. As low pre-embolisation platelet count and advanced maternal age are associated with higher odds of embolisation failure, careful post-embolisation monitoring may be required for such patients. Embolisation also allows subsequent pregnancy. However, further studies are required to assess the outcomes of post-embolisation pregnancies.
INTRODUCTION
Primary postpartum haemorrhage (PPH) is one of the most common causes of maternal morbidity and mortality around the world. Initial management usually involves conservative measures such as fluid replacement, blood product transfusion, uterine massage and uterotonic medications. Traditionally, failure of these conservative measures requires surgical hysterectomy, which negates the possibility of future pregnancy.(1)
Transcatheter pelvic artery embolisation (PAE) is increasingly being accepted as the first-line treatment for patients with primary PPH who are unresponsive to conservative management. As compared to hysterectomy, PAE can potentially achieve haemostasis while preserving the patient’s reproductive capacity. It allows the bleeding site to be identified if localisation cannot be ascertained intraoperatively. Pelvic embolisation is also less invasive and avoids side effects associated with major operation and anaesthesia.(2-4) Furthermore, pelvic embolisation does not preclude surgery if haemostasis is not achieved by embolisation alone.(5)
It is important to be aware of clinical and procedure-related parameters that are associated with embolisation failure and the need for post-PAE surgery. This would allow us to identify patients who are at risk and require closer clinical monitoring or prompt surgical intervention should continuous bleeding arise. To our knowledge, few studies in the literature have focused on this issue in a regional acute hospital setting. Hence, we performed a retrospective study over 12 years to review patients on whom urgent PAE was performed at Pamela Youde Nethersole Eastern Hospital, Hong Kong. The aim was to identify potential predictors of embolisation outcome and the efficacy of embolisation (i.e. its success rate and effects on subsequent fertility).
METHODS
Approval for the study was obtained from the institutional review board and ethics committee. All patients who underwent urgent pelvic embolisation for primary PPH between 1 October 2000 and 31 October 2012 at our centre were retrospectively reviewed. Primary PPH was defined as blood loss > 500 mL from the genital tract within 24 hours of delivery.(6) Data was collected on patient demographics, obstetric history, pre- and post-embolisation blood chemistries and clotting profiles, amount of blood loss, transfusion history, embolisation method, procedure times and embolisation results.
Prior to the procedure, its potential risks and benefits were fully explained to the patients and written informed consent was obtained. Attempts were made to correct systemic coagulation abnormalities before the procedure by using fresh frozen plasma and platelet concentrates. Suitable blood transfusion was also given, if necessary, as well as intravenous prophylactic antibiotics (ampicillin and metronidazole).
Under local anaesthesia, digital subtraction angiography was performed by an experienced vascular interventional radiologist using the femoral arterial approach. Pelvic arteriography was performed, followed by angiography of the bilateral internal iliac arteries to identify the source of bleeding. Under fluoroscopic control, embolisation agents were introduced to obtain satisfactory devascularisation. Post-embolisation angiography was performed for all patients to ensure the occlusion of bleeding vessels (Figs.
Fig. 1
Angiograms of the right internal iliac artery in a patient with primary postpartum haemorrhage who received urgent pelvic artery embolisation. (a) Pre-embolisation angiogram shows contrast extravasation (arrow) over the uterine region. (b) Post-embolisation angiogram showed cessation of contrast extravasation and reduced vascularity.
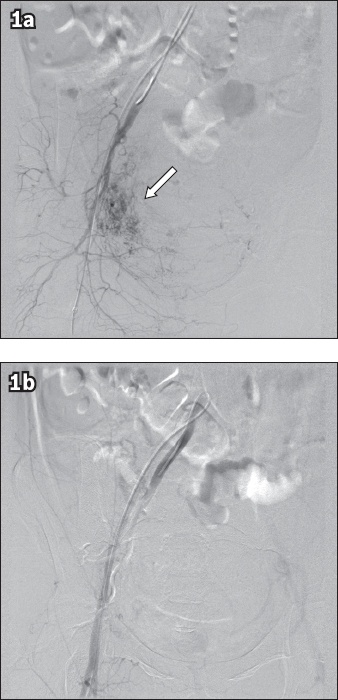
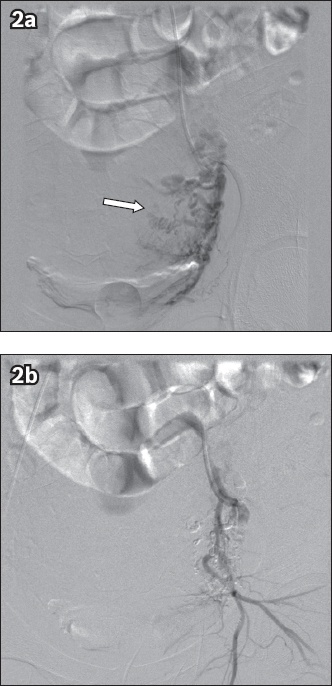
Fig. 2
Angiograms of the left uterine artery in a patient with primary postpartum haemorrhage who received urgent pelvic artery embolisation with gelfoam pledgets. (a) Pre-embolisation angiogram shows hypertrophy of the left uterine artery (arrow). (b) Post-embolisation angiogram shows significant reduction in vascularity in the previously hypertrophied artery.
Technical success was defined as the absence of any angiographic evidence of active contrast extravasation at the end of the procedure. Clinical success was defined as adequate haemostasis being achieved after the first embolisation procedure without the need for subsequent re-embolisation or surgical intervention (including surgical arterial ligation, laparotomy or hysterectomy). Clinical failure of the procedure referred to patients who required repeat embolisation or subsequent surgical intervention for continued bleeding.(7)
Statistical analysis was performed using SPSS version 15.0 (SPSS Inc, Chicago, IL, USA). Testing for normality of data was performed using Shapiro-Wilk test. Student’s t-test, Mann-Whitney U test and Fisher’s exact test were performed to compare peri-embolisation factors between patients with clinical success and those with clinical failure following the first embolisation procedure. A p-value < 0.05 was considered statistically significant.
The potential predictors of clinical outcome were first assessed individually using logistic regression (the ‘enter’ method) for associations with clinical failure. Multivariate logistic regression analysis using the forward and backward stepwise (likelihood ratio) methods was then performed to identify independent predictors and for model development to predict clinical failure. Goodness of fit of the regression model was assessed using the Hosmer-Lemeshow test. As one patient had two separate pregnancies, both of which required PAE, regression analysis was adjusted for any potential confounding of history of prior PPH.
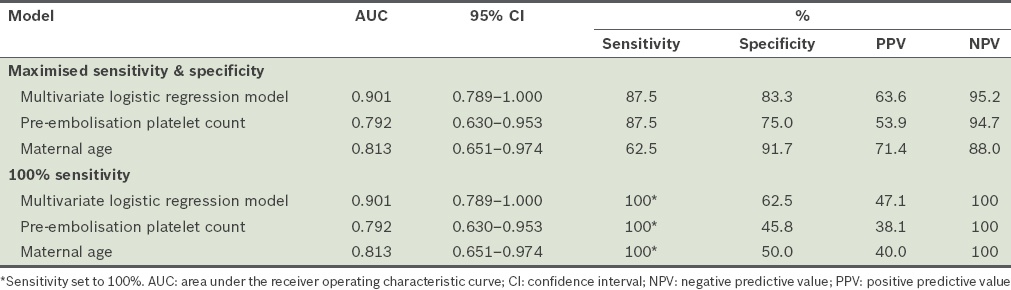
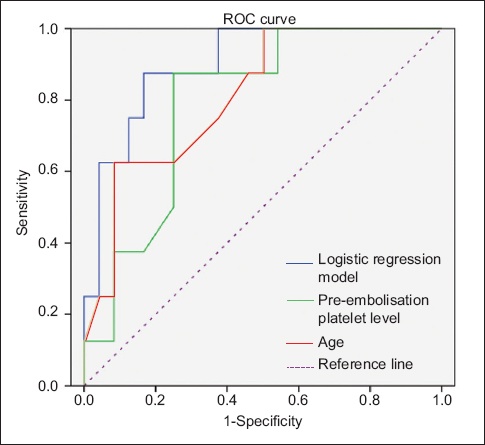
The diagnostic performance of each independent predictor and the overall multivariate logistic regression model were assessed using the area under the curve (AUC) of their receiver operating characteristic (ROC) curves. Sensitivities, specificities, positive predictive values and negative predictive values were calculated.
Table I
Diagnostic performance of regression models.
RESULTS
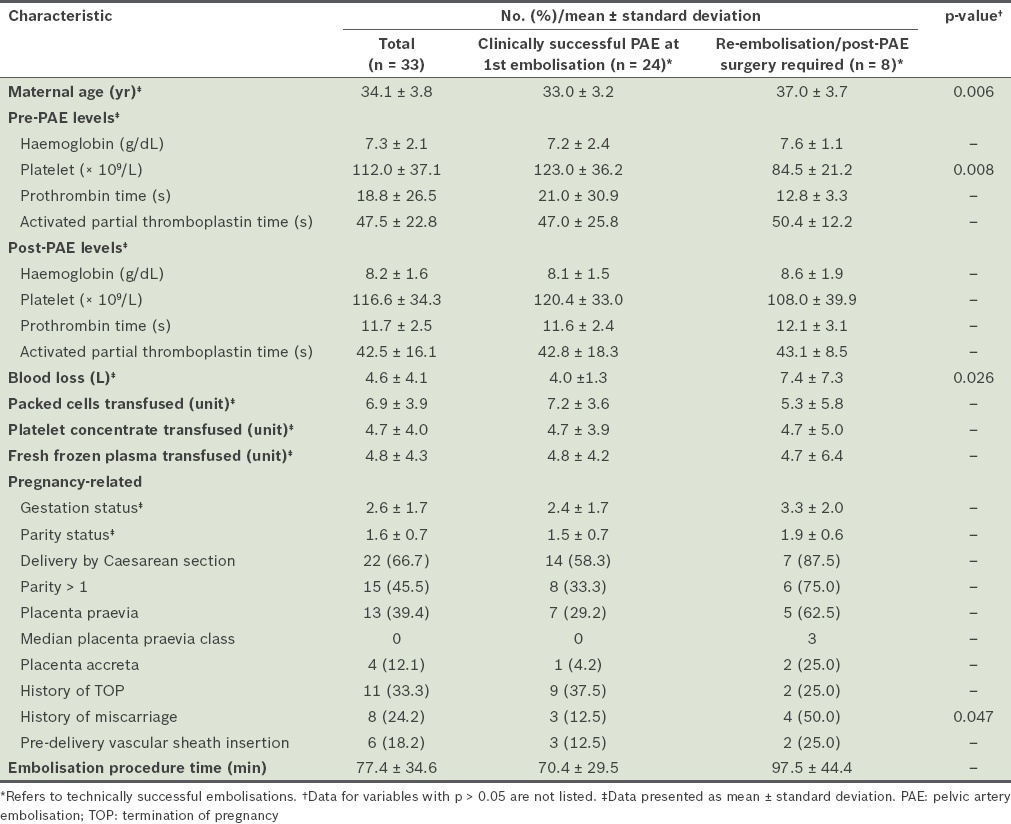
There were 47,221 deliveries in our hospital during the 12-year study period, and urgent PAE was requested for 33 patients with primary PPH (0.07% of total patients, or approximately 1/1,431 deliveries). Patient characteristics are shown in
Table II
Patient characteristics.
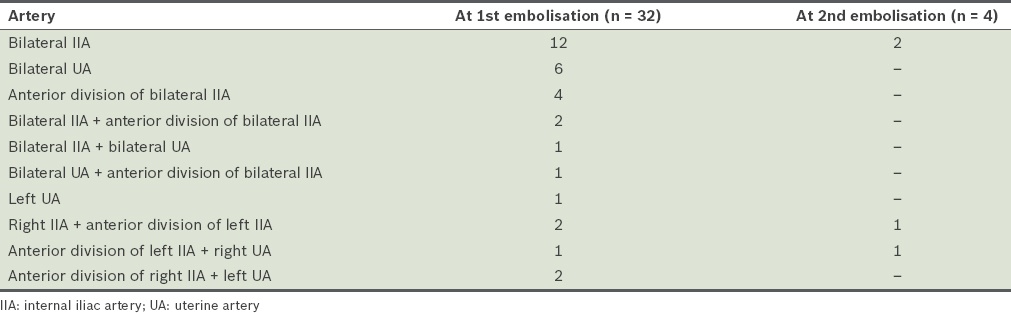
The mean procedure time for embolisation was 77.4 ± 34.6 minutes. Pelvic angiography and bilateral internal iliac angiography to locate the source of bleeding prior to embolisation were performed for all patients. Embolisation was performed using the 5 French C1 catheter in 97.0% (n = 32) of patients. An RC1 catheter was used for the remaining patient. Microcatheters were used for two patients. The arteries that were embolised, which were chosen according to findings noted on angiography, are listed in
Table III
Choice of artery embolised.
The clinical outcomes of the patients are summarised in
Fig. 3
Chart shows the clinical outcome of patients in the study. PAE: pelvic artery embolisation; PPH: postpartum haemorrhage
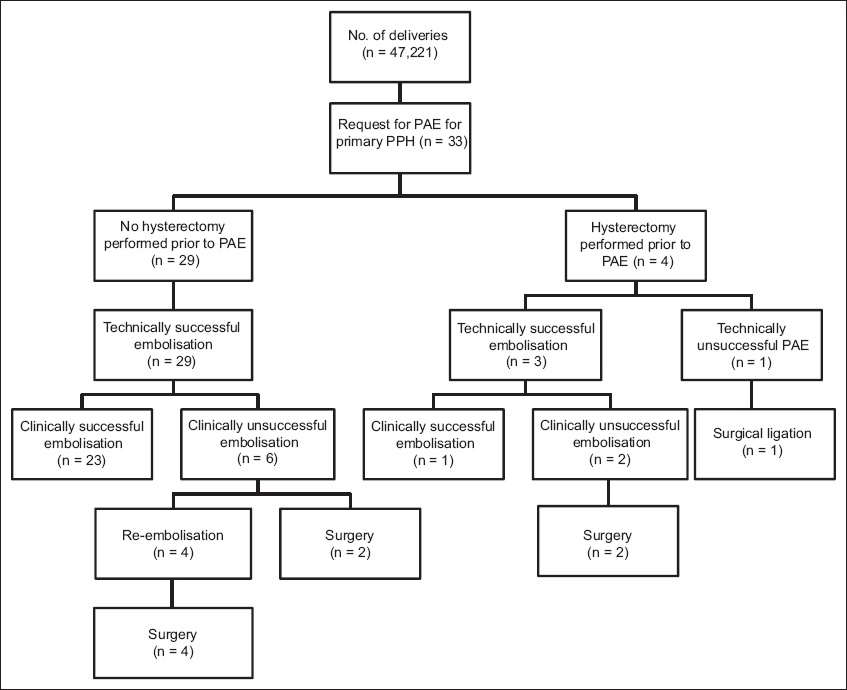
Table IV
Characteristics of patients in whom the first embolisation procedure failed to achieve haemostasis.
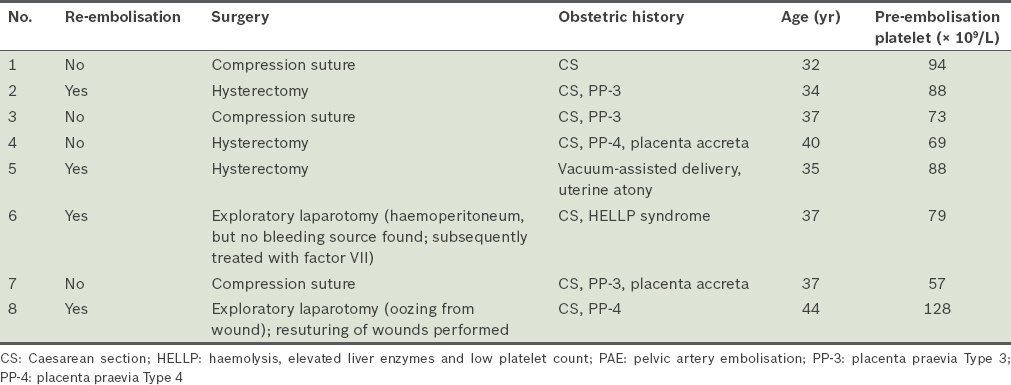
Patients with clinical failure after the first embolisation had significantly lower pre-embolisation platelet counts (p = 0.008), advanced maternal age (p = 0.006), greater blood loss (p = 0.026) and higher likelihood of a history of miscarriage (p = 0.047) when compared to patients with clinical success (
Table V
Logistic regression for individual potential clinical predictors.
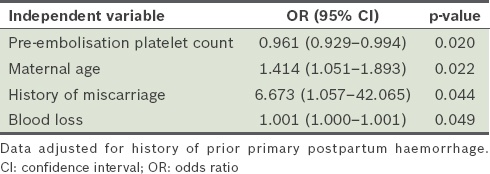
Table VI
Multivariate logistic regression.

The multivariate logistic regression model for the relationship between embolisation outcome and clinical predictors was: Z = (0.395 × age) − (0.051 × pre-PAE platelet count) − 9.802, where Z is related to the probability (p) of embolisation failure according to the equation: p = 1/(1 + e–z). The Hosmer-Lemeshow test showed that the model was well calibrated (p = 0.788).
The diagnostic performance of the multivariate logistic regression model and individual predictors is shown in
Fig. 4
Receiver operating characteristic (ROC) curve shows the diagnostic performances of the multivariate logistic regression model and individual predictors. Diagonal segments are produced by ties.
All patients who did not have pre- or post-PAE hysterectomy (n = 26) resumed normal menstruation after the procedure. The time to return of menstruation ranged from 3–8 months. Two patients had documented pregnancies after pelvic embolisation. The first patient successfully gave birth about 1.5 years after the first PPH pregnancy. In the subsequent pregnancy, the patient was diagnosed with Type 3 placenta praevia antenatally and was admitted for elective Caesarean section. She also had primary PPH during the second pregnancy, which was stopped successfully after a single embolisation procedure. The second patient, who had suffered from HELLP (haemolysis, elevated liver enzymes and low platelet count) syndrome during her first pregnancy, underwent two embolisations with subsequent exploratory laparotomy in view of persistent clinical signs of bleeding. However, no bleeding source was identified and the patient finally stabilised after factor VII was given. This patient conceived again eight years later. However, as the later pregnancy was noted to be ectopic, it was subsequently terminated.
DISCUSSION
Our study showed that pre-embolisation platelet count and maternal age were significant independent predictors of clinical failure of embolisation in women undergoing emergency PAE for primary PPH. This finding supports the belief that disseminated intravascular coagulation (DIC) is an important determinant of embolisation failure, as low platelet count is one of the criteria for DIC. In a recent study of 257 patients, Kim et al showed that DIC was the only significant predictor of clinical embolisation failure. In their study, the presence of DIC was assessed using a combination of results, including platelet count, prothrombin time and fibrinogen level.(9) In contrast, tests for fibrinogen levels were not routinely requested for our patients with primary PPH, due to limitations in availability. Hence, the presence of DIC, according to established laboratory criteria, could not be assessed in our cohort. However, other parameters that are also part of the criteria for DIC, such as platelet count and prothrombin time, were examined in our patients. Our finding that platelet levels were a predictor of embolisation outcome echoes that of previous studies in the literature,(9,10) as platelet count and DIC are intimately related. In addition, when the operative records of our patients with clinical failure of embolisation were examined, it was noted that many of these patients had persistent slow venous oozing from wound sites although no active bleeders were identified. The association between low platelet count and impaired haemostatic response(11-13) explains the continuous venous oozing and clinical deterioration observed in these patients despite their technically successful embolisations, with the absence of contrast extravasation on angiography.
Maternal age was also an independent predictor for clinical outcome of embolisation in our cohort. It is well known that advanced maternal age is associated with haemorrhage during the postpartum period and Caesarean sections.(14,15) Advanced age is also associated with decreased wound healing ability.(16,17) These factors may have contributed to the positive association observed between maternal age and embolisation failure in our patients.
ROC curve analysis showed that the multivariate logistic regression model using pre-embolisation platelet count and maternal age in combination as predictors had a higher capability of predicting the risk of embolisation failure than when the two factors were used independently (
Although other studies have described other potential predictors of PAE failure, including the amount of blood transfused and haemoglobin level,(18,19) these factors were not statistically significant in our study. These earlier studies were also limited by the larger number of predictive factors and the lack of multivariate analysis.(9,20) Cheong et al have recently suggested that the observed correlation between PAE failure and increased blood transfusion may be a result of longer procedure times for patients with clinical failure, rather than increased blood transfusion being a causative predictor of embolisation failure by itself.(20)
In our study, which was performed in a regional acute general hospital setting, urgent PAE showed a success rate of over 70%, with no maternal deaths or complications. Follow-up of our post-PAE patients showed that all clinically successful patients had subsequent return of menstruation, except for the patient who had pre-embolisation hysterectomy. Two patients from our cohort successfully conceived after PAE – one gave birth successfully (although recurrent primary PPH requiring PAE was observed in the second pregnancy as well), while the other patient, who became pregnant again eight years after PAE, had an ectopic pregnancy that was subsequently terminated. A review of the literature reveals that there is a high chance of successful conception after PAE.(7,10,21-23) However, in line with our study, a number of patients with subsequent post-PAE pregnancies were reported to have recurrent PPH; a few had ectopic pregnancies, abortions and miscarriages. Patients who have undergone PAE should therefore be considered to be at elevated risk of recurrent PPH when compared to the general population.(21,22)
In our study, all patients with failed first embolisation eventually required surgery regardless of whether a second attempt at embolisation was made (
With respect to the technical aspects of PAE, in our study, none of the recorded procedure-related parameters (e.g. participating radiologist, the artery embolised, procedure time, embolisation method used and presence of pre-delivery vascular sheath insertion) were significantly associated with embolisation outcome. At our centre, the usual practice for patients who are clinically considered to be at high risk of primary PPH is to undergo pre-delivery vascular sheath insertion. In this study, the number of patients with primary PPH who underwent pre-delivery vascular sheath insertion was quite low (6/33 patients, 18.2%) and no significant associations were noted between embolisation outcome and the presence or absence of pre-delivery vascular sheath insertion. This may indicate a need to review the necessity of pre-delivery vascular sheath insertion for clinically high PPH risk patients and to possibly revise the clinical criteria for patient selection for pre-delivery vascular sheath insertion. Further studies are warranted to assess the actual number of patients receiving pre-delivery vascular sheath insertion, the incidence of primary PPH in such patients and the clinical criteria that can better predict the occurrence of primary PPH in pregnant patients.
To conclude, emergency PAE is an effective measure to arrest life-threatening bleeding in patients with primary PPH and should be considered as the first-line management for those who are unresponsive to conservative measures. Low pre-embolisation platelet count and advanced maternal age are positively associated with the need for subsequent surgical intervention in these patients. Therefore, careful post-embolisation monitoring may be required for patients with primary PPH undergoing PAE. As embolisation allows for subsequent pregnancies, further studies are required to assess the effect of embolisation on the clinical outcome of subsequent pregnancies in these patients.


