Abstract
INTRODUCTION
To study the prevalence of hepatitis C virus (HCV) infection in blood donor (BD), haemodialysis (HD) and intravenous drug user (IVDU) populations in Singapore and assess the IL28B polymorphism if HCV positive.
METHODS
The BD population were healthy volunteers, the HD population were patients who were on haemodialysis for at least six months of follow-up between January 2009 and December 2014. IVDU population was from inmates at halfway houses who consented.
RESULTS
Between 2011 and 2014, of 161,658 individuals who underwent screening prior to blood donation, 95 (0.059%) were positive for HCV. Of the 42 sera available, common genotypes (GTs) were GT-3 (47.6%) and GT-1 (31.0%). Of 1,575 HD patients, 2.2% were anti-HCV positive. The HCV GT distribution was HCV GT-1 (32.4%), HCV GT-3 (20.5%) and GT-6 (8.8%). 83 halfway house inmates were screened. Of the 47 IVDUs, 36.2% were anti-HCV positive with predominant GT-3 (%). IL28B polymorphism was noted to be CC predominantly 85.3%.
CONCLUSION
Prevalence of HCV infection has decreased in both the BD and HD populations. However, it remains high in the IVDU population. GT-1 remains the most common in the HD population; however, GT-3 infection is now more common among the BD population in Singapore. IL28B - CC is the predominant variant among the HCV-infected individuals in Singapore.
INTRODUCTION
Hepatitis C virus (HCV) infection is a global prevailing public health problem, infecting an estimated 130 to 150 million people worldwide.(1) Asians account for approximately 50% of individuals with HCV infection, primarily attributed to the overall population density of this region.(2) Among the more than 1.4 million deaths caused by viral hepatitis, approximately 60% occurred in Asia.(3)
The prevalence of HCV infection varies depending on the geographical location of the country and its socioeconomic status, with less than 2% in developed countries and up to about 15% in developing nations.(2) This heterogeneity is further compounded by the several genotypes and subtypes of HCV that are found throughout the world. Global estimates on the prevalence of chronic hepatitis C (CHC) have been found to be as high as 90% or more in high-risk groups such as intravenous drug user (IVDU) populations.(4)
The importance of understanding the epidemiology, including the distribution of HCV genotypes, is invaluable in establishing important public health policies and future healthcare plans. Different treatment regimens exist for each genotype, with varying response rates to conventional antiviral therapy.(5) Combination treatment with pegylated interferon and ribavirin (PEG-IFN+RBV) has been the conventional therapy for CHC until the recent arrival of direct-acting antiviral (DAA) agents. Although DAA therapy has been shown to be efficacious, its cost remains an impediment for routine use.(6)
The last HCV epidemiology study in the general population of Singapore was conducted in 1995, and the prevalence of HCV infection was found to be 0.37% among the blood donor (BD) population.(7) We hope to determine the prevalence of HCV infection among various populations and certain high-risk groups in Singapore, as well as the prevalence of the IL28B single nucleotide polymorphism, which has previously been shown to be linked with response to therapy.(8) Through this study, we hope to better understand how best to use our limited resources to treat CHC in these populations. The primary objective was to study the prevalence of anti-HCV antibody and the frequency of various HCV genotypes in the following populations: (a) BDs; (b) end-stage renal disease patients on haemodialysis (HD); and (c) the IVDU population. The secondary objective was to study the prevalence of the IL28B human allele polymorphism within the high-risk populations to determine the possible treatment response to conventional versus newer, more expensive therapies for CHC.
METHODS
This was a retrospective cohort study where the sera of the HCV-positive individuals were retrospectively analysed. Due to the heterogeneous nature of the three populations studied, the methods used to assess prevalence in each group are discussed in detail below.
In the BD population, data regarding HCV prevalence was provided by the Health Sciences Authority of Singapore for the period of 2011–2015. As part of the blood donation protocol, all BDs are voluntarily recruited throughout Singapore and every BD undergoes a medical examination and interview as part of the assessment. A quantity of 350–450 mL of blood is collected from each BD. Samples of the BD’s blood are tested for blood-borne pathogens, including HCV. HCV infection is screened using both anti-HCV antibody testing with enzyme-linked immunosorbent assay and HCV nucleic acid testing. If either of the tests is found to be positive, the BD is recalled for counselling and follow-up. In addition, the residual blood serum samples of 40 HCV-seropositive and two HCV nucleic acid testing-positive anonymised BDs collected from 2011 to 2015 were retrieved for further analysis, along with information on age, gender and race.
For the HD population, data collected between 2009 and 2014 was extracted from the database of the Department of Renal Medicine, Singapore General Hospital (SGH), Singapore. The database consists of the records of end-stage renal disease patients who received medical consultation at SGH and underwent regular HD at a neighbouring dialysis centre in the community. Patients who were on HD for at least six months and had HCV serology testing done between 1 January 2009 and 31 December 2014 were included in the study. Patients who started dialysis before 2009 were excluded.
Routinely, all HD patients underwent HCV serology testing every six months while on haemodialysis. Positive HCV serology is defined as a positive enzyme immunoassay test (Architect i2000SR Immunoassay Analyzer, Abbott Diagnostics, Lake Forest, IL, USA), corroborated by supplementary testing with a line immunoassay (INNO-LIA HCV Score, Fujirebio Europe, Belgium). Those who tested positive for HCV RNA were further genotyped using Versant HCV Genotype 2.0 Assay Line Probe Assay (Siemens, Erlangen, Germany) Those who were anti-HCV antibody positive (AB+), based on data from the SGH renal database, were then approached to participate in the study. Informed consent was obtained to determine their HCV genotype (if not known previously) and IL28B genotype (rs12979860 single nucleotide polymorphism [SNP] allele) from their blood samples.
The IVDU population consisted of individuals who had known prior history of drug abuse. The Prisons Halfway House Scheme allows individuals who have a past history of drug abuse to spend the last stage of their detention in an out-of-prison facility prior to their release. Permission was granted for HCV screening of inmates in five of such halfway houses, under the Singapore Corporation of Rehabilitative Enterprises administration. Halfway house residents who volunteered to take part in the study completed a questionnaire about their prior history of drug use and their route of drug administration. Informed consent was taken prior to the administration of the questionnaire. Blood drawn was tested for the presence of HCV antibodies using the point-of-care Assure HCV Rapid Test (MP Biomedicals LLC, Santa Ana, CA, USA), which has a sensitivity and specificity of 98.1% and 98.9%, respectively. The remaining blood samples were transported to a tertiary healthcare institute for formal serology (i.e. enzyme immunoassay test), HCV genotyping and IL28B genotype testing. HCV genotyping was done at the National University Hospital, Singapore, clinical laboratory using a reverse line probe assay (Versant HCV Genotype 2.0 Assay Line Probe Assay) that requires a minimum viral load of approximately 2,000 IU/mL.
HCV-positive samples from both the HD and IVDU populations were tested for their IL28B rs12979860 allele. DNA extraction was performed in accordance with the protocol provided by the Maxwell 16 MDx Research System (Promega, Madison, WI, USA). SNP genotyping analysis was done using Applied Biosystems TaqMan assays (Thermo Fisher Scientific, Waltham, MA, USA). The TaqMan probe and primer sets were selected from the company’s ready-designed product list for IL28B polymorphism rs12979860 sequence:
5-TGAACCAGGGAGCTCCCCGAAGGCG-3 allele C and 5-GAACCAGGGTTGAATTGCAC TCCGC-3 allele T. Polymerase chain reaction was performed using Applied Biosystems 7500 Fast Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). The ABI Prism 7500 sequence detector was used to analyse the SNP allele of each sample.
All statistical calculations were done using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA). This study was reviewed by the Centralised Institutional Review Board in accordance with the guidelines set forth in the Declaration of Helsinki (CIRB number: 2015/3168, 2016/2265).
RESULTS
In the present study, the prevalence of HCV infection in the BD population was 0.058%. For the purpose of comparison with the four-year record of the HD population, the prevalence of HCV infection in the BD population from 2011 to 2014 was calculated and found to be 0.059% (
Fig. 1
Chart shows the prevalence of HCV infection (anti-HCV AB+) among the blood donor (BD) and haemodialysis (HD) populations in 2009–2014.
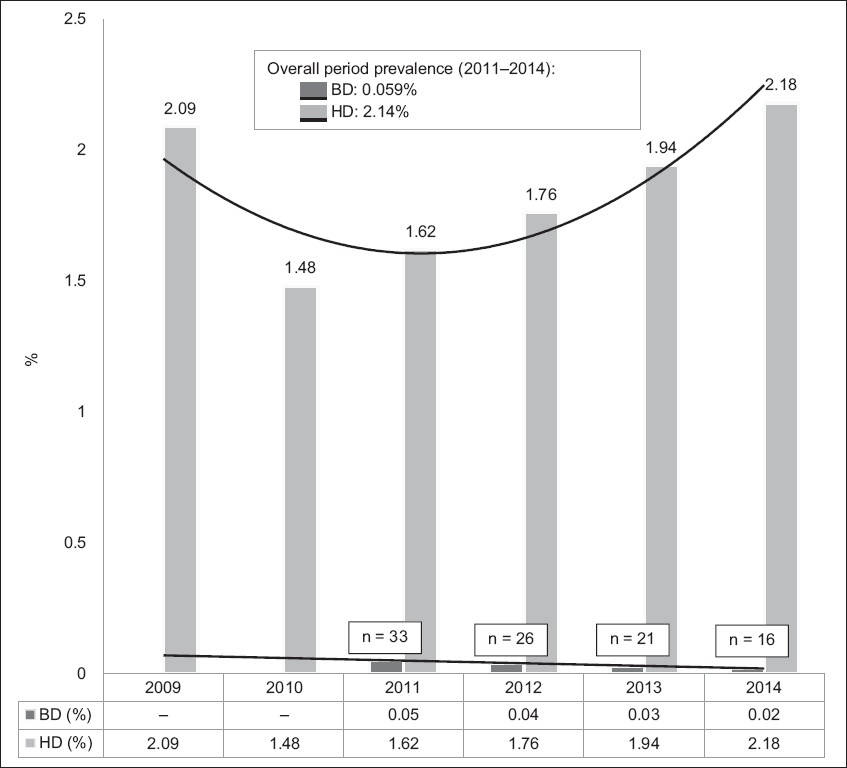
Table I
Demographics of the blood donor population (n = 42).
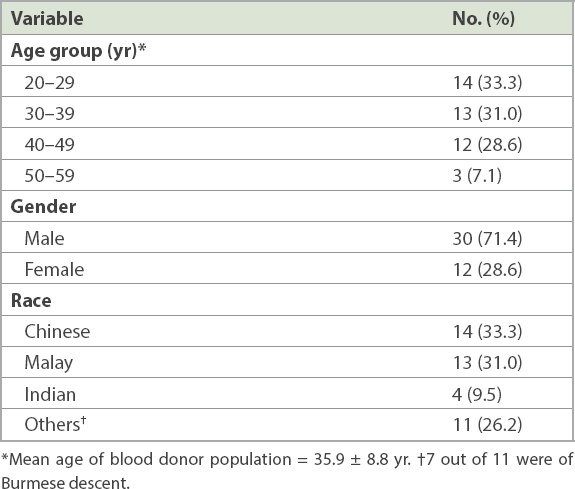
Fig. 2
Chart shows the breakdown of hepatitis C virus (HCV) genotype of the blood donor population according to race (n = 42). *Reported as considerable sequence heterogeneity within the sample.
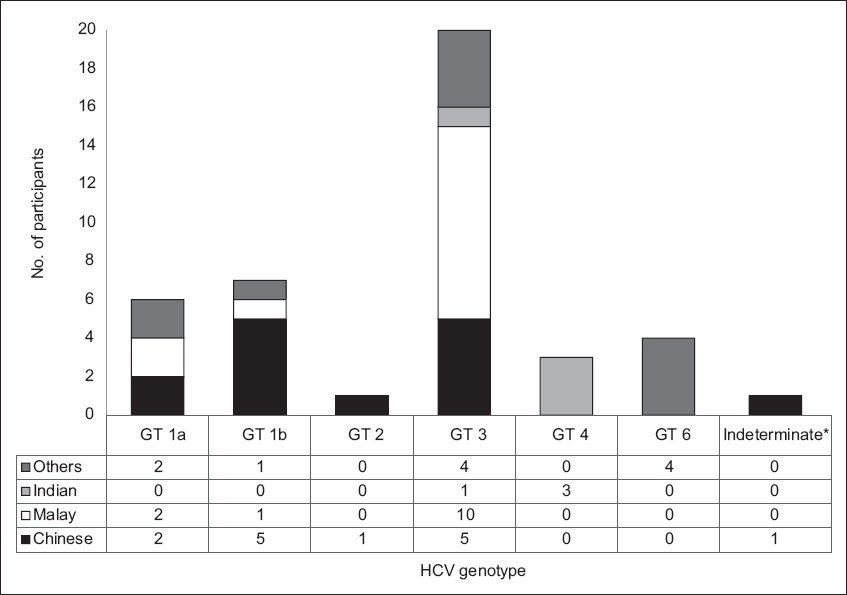
Table II
Demographics of the haemodialysis population.
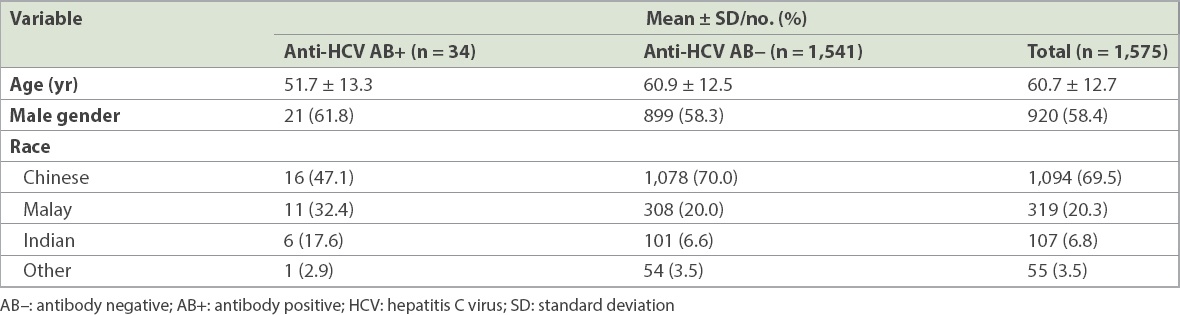
Fig. 3
Chart shows the breakdown of hepatitis C virus (HCV) genotype of the haemodialysis population according to race (n = 34). *Ten patients did not have genotyping done for the following reasons: deceased (n = 4); HCV infection resolved (n = 3); uncontactable (n = 1); rejected study (n = 2).
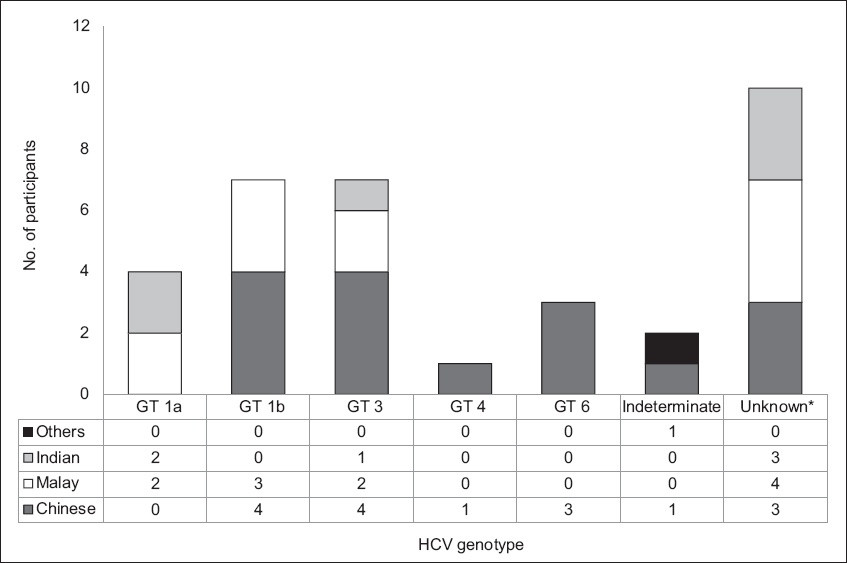
Table III
Demographics of the drug user population (n = 83).
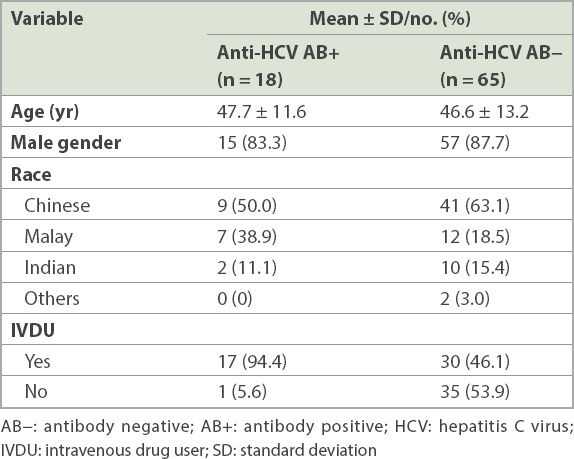
Genotyping could not be done in five of the drug users who were anti-HCV AB+ due to low viral load, i.e. RNA < 2,000 IU/mL (4/18) and insufficient serum (1/18). Of the 13 genotyped specimens, the majority were of GT-3 genotype (8/13) and the remaining were of GT-1a (4/13) and an indeterminate genotype (1/13). Two of the four who had HCV RNA < 2,000 IU/mL had previously been treated for CHC.
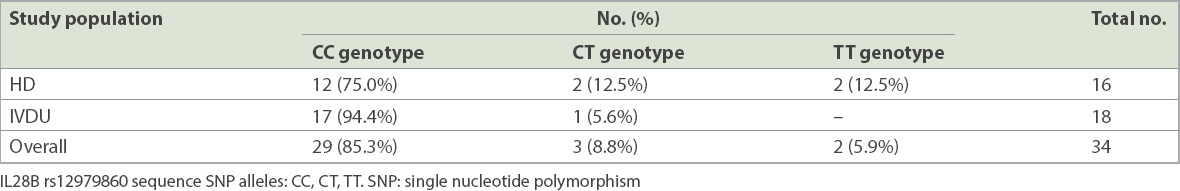
Anti-HCV AB+ blood samples collected from both the HD and IVDU populations were tested for the allelic distribution of IL28B allele rs12979860 (
Table IV
Distribution of IL28B SNP allele in the haemodialysis (HD) and intravenous drug user (IVDU) populations.
DISCUSSION
Previous studies of HCV prevalence in the Singapore general population have put the estimated percentage at around 0.5% in 1995.(7,9-12) The blood bank handled approximately 70,000 BDs in 2015. Current results from BDs have shown a decrease in the prevalence of HCV within Singapore to 0.059% with a decreasing trend between the years 2010 and 2014 (
There is a wide range of HCV prevalence among HD patients worldwide, with a prevalence rate of about 10% in the United States(13) and 4% in Malaysia.(14) The HD population is one of the most at-risk populations for HCV infection.(15) In 2013, the National Registry of Disease, Singapore, reported a HCV infection prevalence rate of 3.7% in the HD population.(16) Our retrospective cohort study showed a prevalence rate of 2.2% (34/1,575) and a relatively stable annual prevalence rate throughout a five-year period (2009–2014). While our study was confined to HD patients who were on follow-up in one tertiary care hospital in Singapore, its large cohort size is able to provide insights on the prevalence of HCV infection among patients receiving HD in the Singapore community.
The lower prevalence of HCV infection in our HD populations, as compared to historical data, is consistent with the progressively decreasing HCV prevalence in our BD population. Implementation and enforcement of infection control strategies, as well as increased blood transfusion screening, have been shown to result in a reduction in blood-borne infections such as HCV,(13,17) and this has probably contributed to the decreasing prevalence in our HD population.
It is interesting to note that, while there is a change in the predominant HCV genotype of the HCV-infected BD population, that in the HD population had remained unchanged, with almost one-third of HCV-infected HD patients showing a GT-1 genotype. Nonetheless, GT-3 infection appears to be increasing in the HD population, as in the BD population.
In 2015, there were 3,343 drug arrests in Singapore.(18) It has been estimated that the prevalence of HCV infection among IVDUs in Southeast Asia is 60%–90%.(4) The role of needle sharing is a known risk factor and cause of HCV transmission among IVDUs.(4) In the current study, almost all who tested positive for HCV infection in the drug abuse cohort had a history of intravenous drug use, yielding a 36.2% HCV prevalence rate. This is comparable to an earlier Singapore study that reported a HCV prevalence rate of 42.5% among those with prior history of intravenous drug use.(19) It is possible that our study could have underestimated the scale of the problem, as it was a short study with a small sample size and participation for HCV screening was offered on a voluntary basis, with a participation rate of 52.9% (89/168). Nonetheless, the genotypes found in our HCV-infected IVDU population were exclusively GT-1a and GT-3, with a predominance of the latter, which was similar to other studies.(20)
As a secondary objective of our study, we looked at the frequency of IL28B rs12979860 allele in our HCV-infected HD and IVDU populations. Our results showed that there was a predominance (85.3% overall) of the favourable CC allele in these populations. Given our findings and the prohibitive cost of DAA agents, and keeping in mind that IL28B SNP is highly associated with increased sustained viral response following conventional PEG-IFN+RBV combination therapy for CHC,(8,21-24) it would seem reasonable to consider IL28B genotyping a priority, with the view of using PEG-IFN+RBV combination therapy as a first-line treatment for CHC, when appropriate, for a substantial proportion of the HCV-infected population in Singapore. Thus, the costly DAA agents can be reserved for the minority who have contraindications to, or poor predictive factors for sustained viral response following PEG-IFN+RBV combination therapy. This is particularly relevant to those with GT-3 HCV infection, such as the majority of the IVDU population, in which the choice of DAA agents is limited.
Our HD database was chosen due to its accessibility. However, this being a population followed up by a single public institution – albeit receiving haemodialysis from different HD centres in our wider Singapore community – has its limitations. Furthermore, our dataset did not allow us to calculate the length of time between the commencement of HD to the time of diagnosis of HCV infection. Future studies could explore the relationships between the risk of HCV infection and the length of time a patient has been on HD in the Singapore context. Our IVDU data was also limited by its small sample size of 47 individuals. This would account for the difference in significant values seen when comparing between unadjusted and adjusted odds ratios.
Similar to many developed nations, with increased blood surveillance and more widespread public awareness, the prevalence of HCV infection in both the BD and HD populations has dropped (0.059% and 2.2%, respectively), as compared to historical data. However, more work needs to be done for the at-risk HD population, as we expect increased morbidity and mortality in this population when infected with HCV.(17) The persistently high HCV prevalence in the IVDU population is an important source of spread of HCV. Education programmes targeted at at-risk groups that highlight dangerous practices leading to the spread of blood-borne infections could play a role. The high prevalence of the favourable IL28B CC genotype in our HCV-infected population suggests that the PEG-IFN+RBV combination therapy remains a viable treatment option in selected situations or individuals.
ACKNOWLEDGEMENT
This study was funded by the departmental fund of the Department of Gastroenterology, SGH.


