Abstract
INTRODUCTION
Oral changes observed during pregnancy have been studied for many years, but their magnitude and frequency have not been stressed upon. This study was undertaken to assess the prevalence of oral lesions during different trimesters of pregnancy and their correlation with salivary pH change.
METHODS
The gingival, simplified oral hygiene, community periodontal and decayed-missing-filled teeth indices were used to assess a total of 120 pregnant women (40 in each trimester group) and 40 nonpregnant women (control group). Salivary pH was measured using a digital pH meter. Presence of any oral lesions was determined via oral examination.
RESULTS
Scores for all indices increased while salivary pH decreased from the control group to the first trimester group, through to the third. Oral lesions were seen in 44.2% of pregnant women. Lesions were seen in 27.5%, 52.5% and 52.5% of women in the first, second and third trimesters, respectively. The percentage of pregnant women with one oral lesion was highest in the second trimester (47.5%), whereas the third trimester had the highest prevalence (17.5%) of two concurrent oral lesions. The incidence of fissured tongue was highest in the first trimester group, and that of gingival enlargement was highest in the third trimester group. In the second trimester group, there was an almost equal incidence of fissured tongue and gingival/mucosal enlargement.
CONCLUSION
Most changes in oral tissues during pregnancy can be avoided with good oral hygiene. Salivary pH could be used to assess the prevalence of oral lesions in the different trimesters of pregnancy.
INTRODUCTION
Many epidemiological studies on the prevalence of oral lesions and dental health status of pregnant women have been conducted worldwide.(1-6) However, data on the prevalence of oral lesions in the different trimesters of pregnancy is scarce. Investigations that have been done in the different trimesters are mainly restricted to dental health status and not many factors have been studied. Therefore, this study was undertaken to assess the prevalence of oral lesions during the different trimesters of pregnancy and their correlation with salivary pH change, and comparing the results against those in nonpregnant women. We also attempted to assess the changes to oral hygiene, caries, gingival and periodontal health statuses, and salivary pH in the different trimesters of pregnancy.
METHODS
The study group comprised a total of 120 pregnant women (40 in the first trimester, 40 in the second, and 40 in the third) between 18 and 35 years old who attended the outpatient clinic of the Obstetrics and Gynaecology department of MM Medical Hospital, India. The control group comprised 40 nonpregnant women in a corresponding age group. The following are the exclusion criteria used in the present study: (a) contributory medical history of pathological conditions and history of drug therapy that may cause oral mucosal lesions and changes in salivary pH; and (b) smoking/tobacco chewing habit. Data pertaining to education level and general awareness of oral hygiene were collected. The participants were from nearby villages, and most of them had dropped out of school with only a primary education. The following indices were used to assess participants’ gingival and periodontal health statuses, and determine the number of teeth that were decayed, missing or filled: (a) gingival index (GI);(7) (b) simplified oral hygiene index (OHI-S);(8) (c) community periodontal index (CPI);(9) and (d) decayed-missing-filled teeth index.(10)
Participants were clinically examined for the following parameters: oral hygiene, gingival health, periodontal health, dental caries, and the presence of any gingival or oral mucosal lesions (or any other oral lesions). Saliva samples were collected for the measurement of salivary pH using a digital pH meter (LT-23; Labtronics, Panchkula, Haryana, India). Saliva samples were collected from each participant at least one hour after breakfast, which would yield unstimulated saliva. For the collection of these samples, all participants were requested to be as quiet as possible. They were instructed to allow the saliva to flow into the mouth as normally as possible and expectorate into the large test tubes provided. In all, 8–10 mL of sample was collected for each participant. The pH of each sample was determined within the first half an hour after collection.
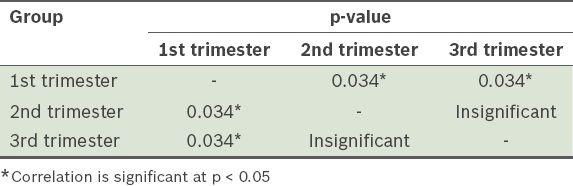
The data obtained was systematically tabulated and subjected to statistical analysis. Statistical correlations among all the parameters within the first, second and third trimester groups and the control group were determined using Pearson’s coefficient correlation. Chi-square test was used to determine differences in the prevalence of oral mucosal lesions between the first, second and third trimester groups.
RESULTS
Scores for all the parameters progressively increased from the control group, through the first and second trimester groups, to the third trimester group, but mean salivary pH progressively decreased from the control group, through first and second trimester groups, to the third trimester group (
Fig. 1
Graph shows an increase in scores for all the parameters examined from the control group to the first trimester group, through to the third trimester group. There was a decrease in mean value of salivary pH from the control group to the first trimester group, through to the third trimester group. DMFT: decayed-missing-filled teeth
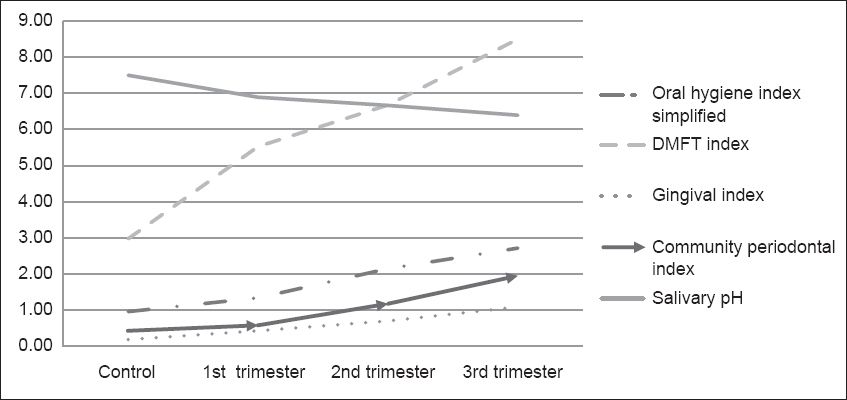
Table I
Distribution of mucosal lesions in the three different trimester groups and the control group.
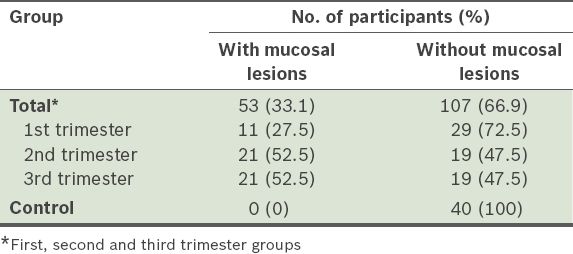
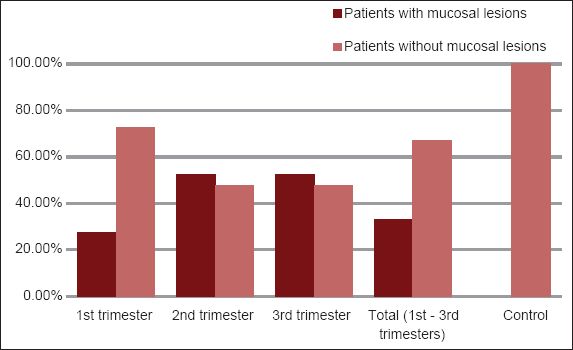
Fig. 2
Graph shows the percentage distribution of mucosal lesions in the different trimesters of pregnancy.
Chi-square comparison of the differences in oral mucosal lesion prevalence among the different trimester groups (
Table II
Comparison of differences in oral lesion prevalence between the first, second and third trimester groups.
Overall, 25% (n = 10), 47.5% (n = 19), and 35.0% (n = 14) of the women in the first, second and third trimester groups, respectively, had one oral lesion, whereas 2.5% (n = 1), 5.0% (n = 2) and 17.5% (n = 7) of the women in the first, second and third trimester groups, respectively, had two concurrent oral lesions (
Fig. 3
Graph shows the percentage distribution of the number of oral lesions.
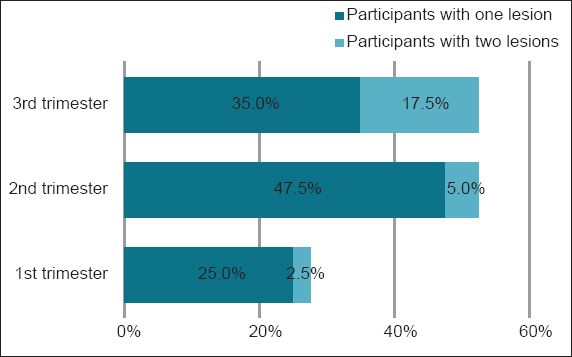
Fig. 4
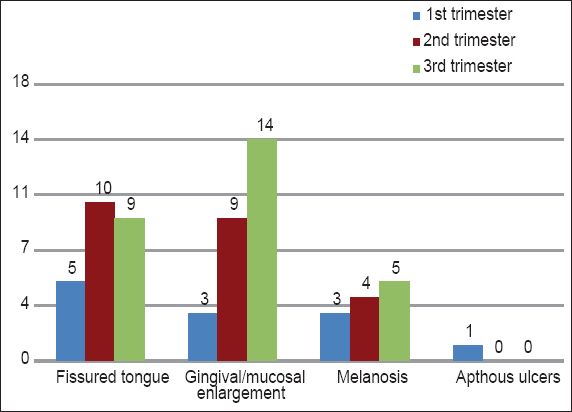
Graph shows the distribution of the type of oral lesions in the different trimester groups.
DISCUSSION
Major physiological and hormonal changes occur during pregnancy. These changes also have far-reaching systemic effects that extend beyond the reproductive system.(1) There are many epidemiological studies on pregnancy worldwide, but few studies discuss the occurrence of oral lesions during pregnancy. The oral mucosa is sensitive to many systemic changes within the body, be they physiological, metabolic, hormonal or chemical. Some of the most frequent and important pathological conditions of the oral cavity are strongly dependent on salivary pH changes.(11) Physiological alterations of saliva during pregnancy may play a role in the pathophysiology of oral conditions seen during pregnancy.(2)
The present study was designed to assess the number and type of oral lesions, and changes in salivary pH in the different trimesters of pregnancy. Changes in the parameters examined (i.e. oral hygiene, dental caries, gingival health and periodontal health) in the first, second and third trimester groups were also assessed using the respective indices. The ages of the participants ranged from 18 to 35 years.
In the present study, oral hygiene in pregnant women was found to deteriorate progressively and gradually from the first to second to third trimester groups. Differences between all the study groups (except between the control group and the first trimester group) were found to be statistically significant. Deterioration in oral hygiene status with an increase in mean OHI-S scores from the first trimester group through to the third has also been observed in many other studies.(3,4) These differences in oral hygiene status could be due to a decrease in oral hygiene practices as the pregnancy advances, as was observed in the present study. An increased incidence of dental caries was seen toward the last months of pregnancy – differences were statistically significant between the first, second, and third trimester groups, and the control group, except between the first and second trimester groups. Similarly, a higher incidence of caries in pregnant women than in nonpregnant women has been seen in different studies.(4,5) Increased levels of Streptococcus mutans and Lactobacillus are found in late pregnancy, which may be the reason for the higher incidence of dental caries in the third trimester group, as observed in the present study.(6) Also, a decrease in oral hygiene practice during pregnancy was observed in a majority of the pregnant women in the present study, which may explain bacterial accumulation during pregnancy. Dietary changes in early pregnancy, such as the regular consumption of sugary snacks and drinks to satisfy cravings or to prevent nausea, may cause a drop in salivary pH.
In our study, pregnant women exhibited higher levels of gingivitis and periodontitis than nonpregnant women. There were statistically significant differences found between all the groups in this study, except between the control group and the first trimester group. A significantly higher mean gingival score was also seen in women in the third trimester in another study.(12) Gingivitis and periodontitis observed in pregnancy are a result of decreased oral care in pregnant women. The effects of certain hormones during pregnancy may predispose pregnant women to gingival inflammation and periodontitis. For example, progesterone is known to lead to the development of localised inflammation.(13,14) A higher incidence of periodontitis has been seen in pregnant women than in those who were not pregnant; this incidence was found to progressively increase from the first trimester through to the third.(7,15) A probable reason for this increase could be due to the increased predisposition of periodontal tissues with increasing hormonal levels towards the end of the third trimester.(16)
Changes in salivary pH were in contrast to all the examined parameters in the present study (oral hygiene, dental caries, gingival health and periodontal health). Salivary pH was observed to progressively decrease from the control group to the first trimester group, through to the third trimester group. The differences between salivary pH were statistically significant between all the groups. A lower salivary pH in pregnant women, compared to nonpregnant women, has also been seen in other studies.(17,18)Bicarbonates serve as the primary buffers of saliva and as the bicarbonate level in saliva decreases during pregnancy, a decrease in pH occurs.(19) Regular episodes of vomiting, consumption of more citrus fruits and an increase in the amount of acid-producing, cariogenic bacteria could be the other possible reasons for the decrease in salivary pH.
The following correlations between various parameters in the different trimesters were found:
-
There was worsening of the periodontal condition with the decrease in salivary pH in the third trimester. Decreased oral hygiene practices with localisation of an increased amount of anaerobic bacteria from the saliva in periodontal tissues can induce an aggravated inflammatory response.(6)
-
Together with an increase in OHI-S scores, a progressive increase in GI scores in all the sample groups was seen. During pregnancy, gingiva becomes less efficient at resisting inflammatory changes due to local irritants such as bacteria and dental plaque. With the accumulation of local irritants due to the poor oral hygiene observed in our study participants, the incidence of gingivitis and periodontal destruction was found to increase in all the sample groups.
-
Simultaneous increases in the incidences of periodontitis and gingivitis were seen in all the sample groups. This could also be due to an aggravated response of the periodontium, as well as the damaging processes that accompany periodontal disease, which is associated with local irritants like dental plaque.(14)Other possible additional factors are: (a) the effects of hormones on gingival vasculature; (b) subgingival microbiota; (c) specific cells of the periodontium; and (d) the local immune system during pregnancy.(20)
-
With the decrease in salivary pH observed in the pregnant women in our study, a significant increase in OHI-S was seen in the first trimester group, but this increase was not significant in the second and third trimester groups. The increased activity of cariogenic microflora, along with an increase in dental plaque accumulation, leads to greater acid production, thereby leading to a decrease in salivary pH in pregnancy.
-
In all the sample groups, a decrease in salivary pH was observed with an increase in caries incidence. A decreased concentration of carbonic anhydrase enzyme (salivary buffer) has been seen to lead to an increased prevalence of caries.(21)
-
A simultaneous increase in gingivitis was seen with the decrease in salivary pH in the first, second and third trimester groups. However, this was not seen in the control group. This increase in gingivitis could be due to an aggravated response of the gingiva to the presence of increased localised irritants (e.g. bacteria), which increases at low salivary pH values.(6)
The prevalence and assessment of the number and type of mucosal lesions in the different trimester groups were as follows:
-
Mucosal lesions were present in 44.2% of the pregnant women. A higher incidence of lesions is typically seen in pregnant women than in nonpregnant women.(22) Gingivitis and periodontitis result in the increased vascularity of the gingiva; both facilitate the action of local irritants (e.g. trauma or bacterial plaque), and thus contribute to the development of oral lesions during pregnancy.(23)
-
A higher prevalence of oral lesions (52.5%) was seen in both the second and third trimester groups, as compared to the first trimester group (27.5%). This increase may be related to the increasing severity of gingival and periodontal changes during the second and third trimesters, with gingival and periodontal tissues more vulnerable to the action of local irritants as plasma progesterone and oestrogen levels peak by the end of the third trimester.(16) Oestrogen also decreases keratinisation and results in the diminution of the effectiveness of the epithelial barrier.(24)
-
The number of participants with one oral lesion was higher in the second trimester group (47.5%) than in the first (25.0%) and third (35.0%) trimester groups. The number of participants with two oral lesions increased from the first trimester group (2.5%) through to the second (5.0%) and third (17.5%) trimester groups. In all the pregnant women in our study, the number of participants with two oral lesions was less than those with one oral lesion.
-
Fissured tongue was the most common type of oral lesion observed in the first and second trimester groups, followed by gingival/mucosal enlargement and melanosis. In the third trimester group, however, gingival/mucosal enlargement was the most commonly observed oral lesion, followed by fissured tongue and melanosis. Aphthous ulceration was observed in only one participant in the first trimester group. Fissured tongue could be associated with the nutritional deficiencies that are commonly present in early pregnancy.(25) These fissures serve as the protective harbours of bacteria. There could thus also be a possible association between bacterial increase during pregnancy and the incidence of fissured tongue. Gingival/mucosal enlargements and melanosis could be due to changes in hormonal levels during pregnancy. The pathomechanism of aphthous stomatitis is unknown, but immunologic factors may be involved.
The present study was undertaken to assess the changes in oral environment during the different trimesters of pregnancy and to compare these changes against those in nonpregnant women. We drew several conclusions. We found that oral health deteriorated during pregnancy, with it being the worst in the third trimester, as evidenced by the increased prevalence of oral mucosal lesions, dental caries, gingivitis and periodontitis and reduction in oral hygiene status in the third trimester group. Salivary pH was also found to be decreased during pregnancy, with the saliva of pregnant women observed to be more acidic as pregnancy advanced from the first to the third trimester. When compared to nonpregnant women (seeing as salivary pH decreased in pregnancy from the first trimester to the third trimester of pregnancy), we found an increased incidence of dental caries and a deteriorated oral hygiene status, with an increase in dental plaque accumulation, gingivitis and periodontitis. It can thus be concluded that most of these effects on oral tissues during pregnancy could be avoided by practising good oral hygiene. Through our study, we have also found that salivary pH correlated well with the prevalence of oral lesions in the different trimesters of pregnancy. Therefore, we suggest that salivary pH could be used to assess the prevalence of oral lesions.


