Jane, a seven-month old Chinese girl, was taken to your clinic for a high fever (39.6ºC) of two days’ duration for no apparent reason. Jane was born full-term with a birth weight of 2,800 g. Her mother had reported during her postnatal visits that she was coping well. Jane was her first child. However, her mother said that Jane had been refusing her cereals for the past two days and was now taking only half her usual milk portions. On direct questioning, she reported that Jane’s diapers were not as heavy as usual, and her urine had a stronger smell. No blood was noted. Jane seemed more tired but was still interested in playing with her toys about an hour after taking syrup paracetamol self-administered by her mother. On examination, Jane was febrile at 38.9ºC. Her vital signs were stable, her tongue was moist and she had good skin turgor. She was fretful but consolable when carried by her mother. A head-to-toe examination did not reveal any localising signs of infection.
WHAT IS URINARY TRACT INFECTION?
Urinary tract infection (UTI) is defined as the presence of pathogenic micro-organisms in the urinary tract, resulting in a symptomatic inflammatory response, usually evidenced by pyuria.(1) Lower UTI (otherwise known as cystitis) is isolated to the bladder and is the most common form of UTI. Children with lower UTI tend to present with lower tract symptoms, including frequency, urgency, dysuria, and suprapubic pain and tenderness. Upper UTI involves the renal parenchyma, causing acute pyelonephritis (APN). The majority of febrile UTIs in infants and young children are upper UTIs.
Defining the location of the UTI (i.e upper or lower) or as complicated versus uncomplicated is useful in determining prognosis, treatment and follow-up.(1) Complicated UTI involves risk factors that are summarised in
Box 1
Risk factors for urinary tract infection:
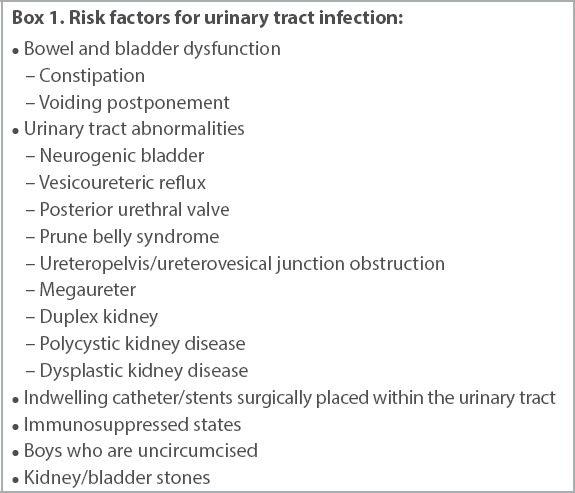
Other features of atypical or complicated UTI include patients who: have a history of previous UTI; are septic or who have a disease that is more severe than usual; have poor urine output or raised creatinine; have a palpable abdominal mass/bladder; fail to respond to appropriate treatment; or have atypical pathogens.(2)
HOW RELEVANT IS THIS TO MY PRACTICE?
UTI is one of the most common bacterial infections seen in children and a leading cause of acute sepsis in infants requiring hospitalisation. It is most common in the first year of life, with a higher incidence in boys compared to girls, in those aged below six months. By seven years of age, 8.4% of girls and 1.7% of boys would have had one or more symptomatic UTIs.(3) UTI carries an acute risk of morbidity and mortality due to urosepsis, renal abscess formation and acute kidney injury. Acquired renal scars can lead to chronic renal insufficiency, proteinuria and hypertension subsequently in adulthood. Different studies show widely varying results on renal scarring following UTI, ranging from 10% to 40%.(4-6)
Fever in a young child is a common presentation in primary care. As UTIs commonly present with acute undifferentiated fever, especially in a pre-verbal child, it is important that first-line healthcare providers appropriately diagnose and treat UTIs in children promptly, given their immediate complications as well as potential for permanent renal damage with considerable sequelae.
WHAT CAN I DO IN MY PRACTICE?
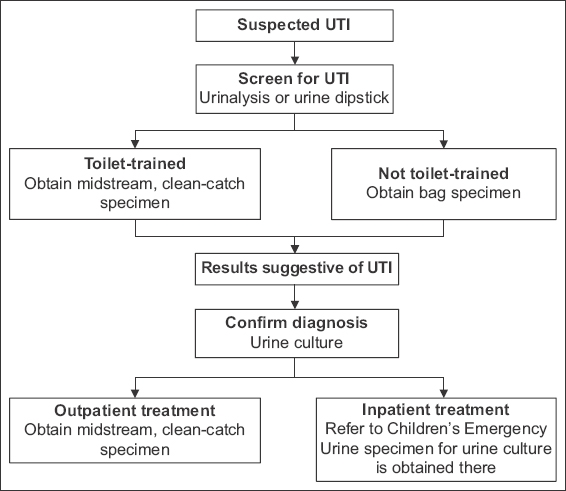
Recognising and treating UTIs promptly and accurately is important. Due to the non-specificity of symptoms of UTI in young children, screening for UTI should be done early via urine investigations, especially in the absence of an obvious source of infection. A properly collected urine sample should be sent for culture before starting treatment.
Fig. 1
Flowchart shows workflow for diagnosing urinary tract infection (UTI).
History and physical examination
Clinical signs and symptoms of a UTI depend on the age of the child, location of the UTI (upper or lower UTI) and severity of the illness. Fever is not a feature in lower UTIs, which tend to present with local or voiding symptoms such as dysuria, frequency, urgency and suprapubic pain. Important points in history taking and physical examination are summarised in Tables
Table I
Important aspects of history taking for children with UTI.
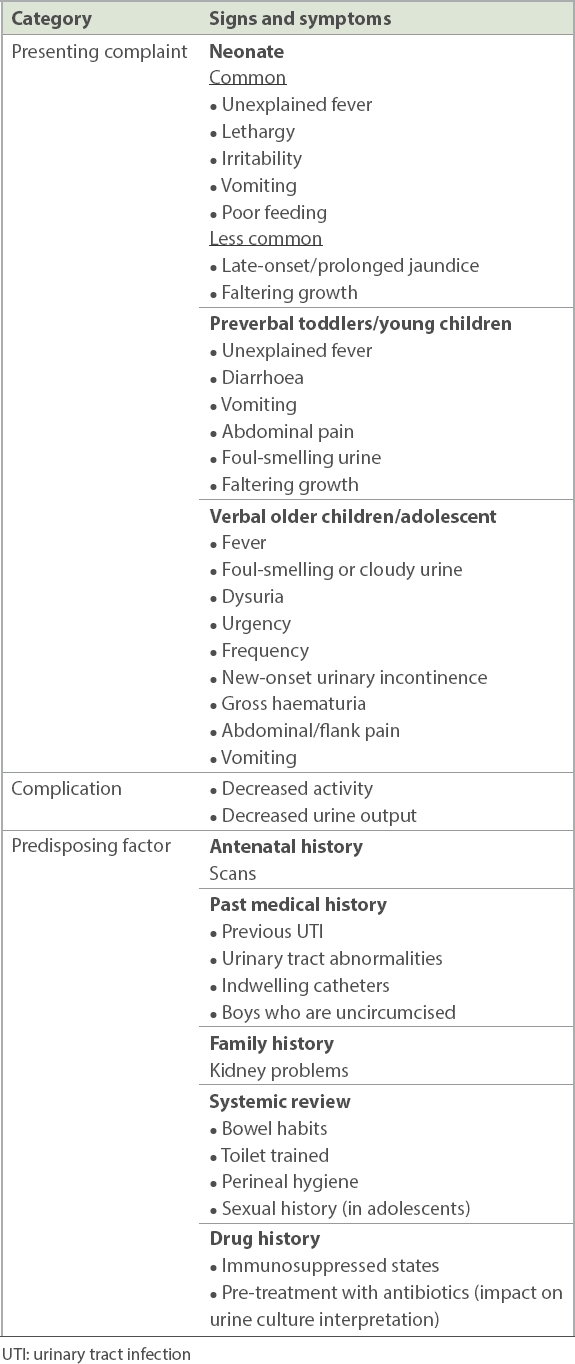
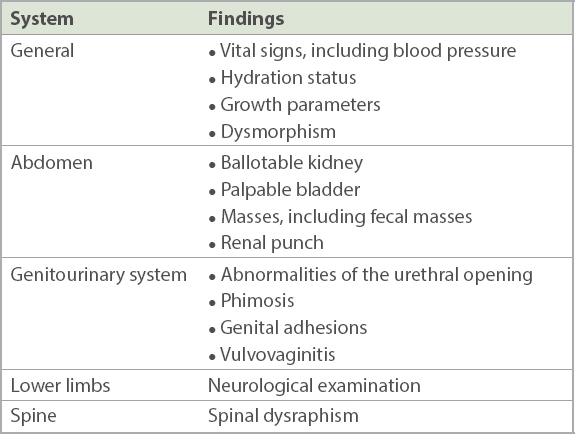
Table II
Important aspects of physical examination for children with urinary tract infection.
The cardinal symptom of APN is high fever without an obvious source of infection, often with chills and rigors. Neonates often present with very non-specific symptoms such as unexplained fever (may be low grade), lethargy, irritability, vomiting or poor feeding. Less commonly, they can present with late-onset/prolonged jaundice or faltering growth. Screening for UTI is part of the workup for neonates or infants with prolonged jaundice and failure to thrive. In pre-verbal infants and toddlers, the presentation is also likely to be non-specific, including unexplained fever, diarrhoea, vomiting, abdominal pain, foul-smelling urine or faltering growth. In older (verbal) children and adolescents, symptoms and signs are more specific, including fever, dysuria, foul-smelling or cloudy urine, urgency, frequency, new-onset urinary incontinence, gross haematuria, abdominal or flank pain, and vomiting. According to guidelines from the American Academy of Pediatrics, factors that increase the likelihood of UTI include age of below 12 months, fever for more than 48 hours, no other apparent source of fever, and fever of or exceeding 39ºC.(7)
In history taking and physical examination, it is important to look for complications as well as predisposing factors for UTI. Acute complications include sepsis, renal abscess formation and acute kidney injury. A history of decreased urine output is significant and could be due to dehydration or acute kidney injury. On physical examination, physicians should look out for the child who has an ill appearance, is mottled, and has unstable vital signs (as tachycardia is the first sign of compensation in a child in shock) and decreased activity. Renal punch, usually performed in older children, may be positive in APN.
Predisposing factors of UTI in children include bowel and bladder dysfunction, urinary tract abnormalities, indwelling catheters, immunosuppressed status, being an uncircumcised boy, and a history of renal or bladder stones (
The term bowel and bladder dysfunction is used to describe the spectrum of lower urinary tract symptoms that accompany bowel disturbance in the form of constipation and/or encopresis. It has been reported to be a key risk factor for recurrent UTIs in children.(8) Bowel habits should be taken as part of history taking. Fecal masses may be noted on physical examination in a child with constipation. Other predisposing factors include inappropriate perineal hygiene, inadequate fluid intake, and infrequent or incomplete bladder emptying. In adolescent patients, it may be appropriate to ask for their sexual history.
Investigations
Urine culture is the gold standard for diagnosing UTI.(9) In patients suspected of having a UTI, a screening urine dipstick test or urinalysis should be done. The technique for urine collection is primarily determined by whether the patient is toilet trained. In children who have not achieved urinary continence, this can be done using a urine bag. In toilet-trained children and adolescents, midstream clean-catch urine samples should be collected. If the results are suggestive of UTI with the presence of pyuria and/or nitrites, a properly collected urine sample should be sent for culture before starting treatment. If the child is clinically unwell, he/she should be referred to the emergency department, where urine specimens may be collected via catheterisation. If the child is otherwise stable, a clean-catch specimen may be obtained in the outpatient setting. As there can be a risk of culture contamination from clean-catch specimens, two separate specimens should ideally be obtained, with at least one specimen obtained prior to initiating antibiotics.
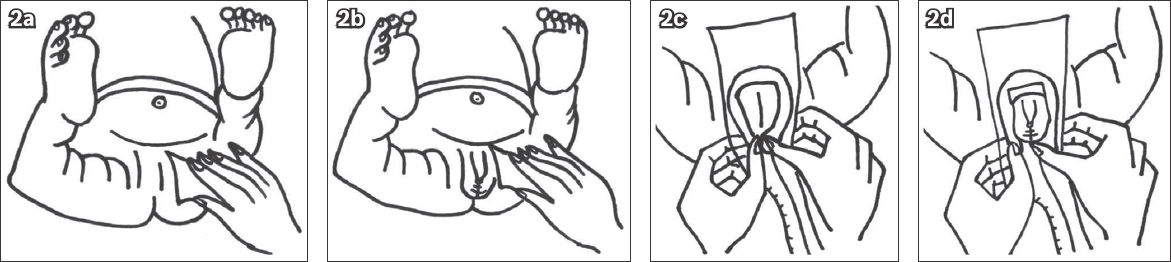
To collect a bagged specimen or midstream clean-catch specimen, the genitalia and perineum should be broadly cleaned with an antiseptic solution to decrease the rate of contamination (Figs.
Fig. 2
Illustrations show the genitalia and perineum being cleaned with an antiseptic solution before obtaining a urine specimen. (a) For girls, the labia should be spread and the perineum cleansed 2–3 times with non-foaming antiseptic solution or mild soap. (b) For boys, the meatus should be cleansed 2–3 times with non-foaming antiseptic solution or mild soap. The foreskin should be retracted before cleansing for those who are uncircumcised. Ensure that the foreskin is reduced to its normal position so that a paraphimosis does not develop. (c & d) A urine bag is applied by removing the backing from the adhesive and applying it over the vulva or penis and scrotum. The bag is regularly monitored and removed once urination has occurred.
It is important to clearly label the method used for urine collection, as the diagnostic criteria may vary according to the method used (
Table III
Method of urine collection and interpretation.
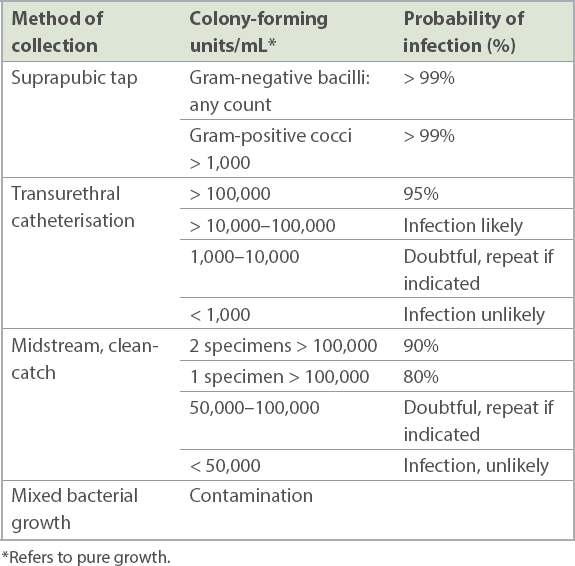
Urine cultures should always be interpreted with clinical correlations. False positive urine culture can be due to contamination, while false negative results can be due to partial treatment or diluted urine. Up to 9% of patients with APN may have equivocal or negative urine culture results.(10) If urine cultures are negative or equivocal but suspicion of UTI is high, repeat urine cultures should be sent and the child referred to a tertiary hospital for further imaging. Viruses, urinary schistosomiasis and funguria are differentials in children with an appropriate history of exposure and high suspicion of UTI but negative urine cultures.
Treatment and follow-up
Timely antibiotic treatment is the cornerstone of UTI treatment and paramount in minimising complications. Most UTIs are caused by Escherichia coli and other enteric Gram-negative organisms, the majority of which are sensitive to gentamicin or cephalosporins. In younger infants (particularly in those aged < 3 months), Gram-positive cocci may cause UTI and thus ampicillin/amoxicillin may be added to cover for these organisms.(11)
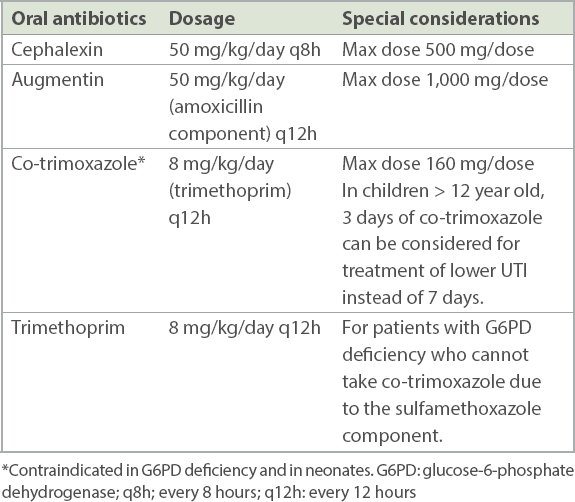
Lower UTIs can be treated in outpatient settings with one week of oral antibiotics such as cephalexin or Bactrim, which is contraindicated in G6PD (glucose-6-phosphate dehydrogenase)-deficient patients.(12) Urine cultures should be actively traced to enable targeted antibiotic therapy, and the patient should be reviewed for resolution of symptoms.
Upper UTIs can be effectively treated with oral antibiotics in the outpatient setting for 14 days or inpatient with intravenous antibiotics, followed by oral therapy once the patient improves.(13) The decision on outpatient or inpatient therapy depends on a few factors. Inpatient parenteral therapy should be considered in acutely ill children, children who cannot tolerate oral therapy and those with complicated UTIs (
Box 2
Indications for inpatient treatment:
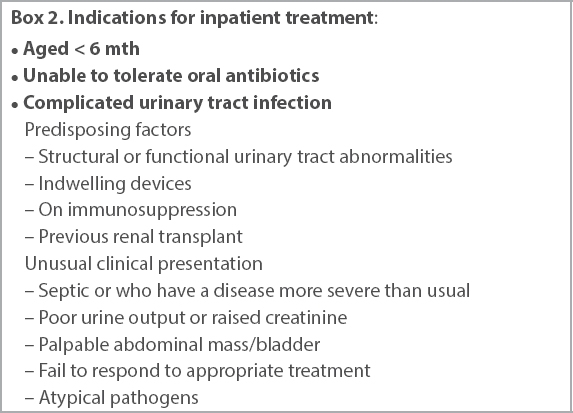
Table IV
Commonly used oral antibiotics and recommended dosing for uncomplicated urinary tract infection (UTI).
Referring to a specialist
As a general rule, all patients with one episode of febrile UTI (upper UTI) and patients who have recurrent cystitis should be referred to a specialist clinic for further evaluation, ideally 4–6 weeks after infection.
Renal and bladder ultrasonography is non-invasive and can be used to evaluate renal parenchyma, renal size and urinary tract anatomical abnormalities. It is recommended after the first febrile UTI. To avoid false positive studies, it is usually done after the UTI has been treated, because acute infection can alter the size and echogenicity of the renal parenchyma and cause transient hydronephrosis.(7) Renal and bladder ultrasonography may be performed during the acute infection to look for renal abscess or pyonephrosis in patients who fail to respond to appropriate treatment or whose illness is more severe than expected. Dimercaptosuccinic acid (DMSA) imaging is a nuclear imaging study used to identify alterations in radiotracer uptake within the renal parenchyma. It is done 4–6 months post infection and is the gold standard for diagnosing renal scars. Micturating cystourethrogram (MCU) is the gold standard for diagnosing vesicoureteric reflux (VUR). It also detects abnormalities of the lower urinary tract. Indications for an MCU generally include atypical or complicated UTI, abnormal renal and bladder ultrasonography, and recurrent UTI.(7)
Secondary prevention
The role of antibiotic prophylaxis in paediatric UTI prevention has been widely debated over the past few decades. In the past, antibiotic prophylaxis was used to reduce the risk of recurrent UTIs and the subsequent developmental of renal scarring, which may in turn lead to renal dysfunction. However, recent studies have shown that although antibiotic prophylaxis may reduce the incidence of recurrent UTI, it did not reduce the risk of or protect against renal scarring. In addition, exposure to prophylactic antibiotics has been shown to increase the likelihood of resistant infections.(3) Locally, in view of some benefits shown in select patient populations, we usually start antibiotics prophylaxis in patients at risk of recurrent UTI, including those with underlying urinary tract abnormalities.
Treatment of constipation with stool softeners has been shown to effectively prevent recurrent UTI in children without urinary tract anatomical abnormalities.(14) Biofeedback training together with regular voiding has been successful in reducing recurrent UTIs in children with detrusor instability or other dysfunctional voiding.(15)
Routine circumcision in boys does not reduce the risk of UTI enough to justify the risk of surgical complications. However, it may considered for boys who have recurrent UTI or high-grade VUR in whom circumcision may be clinically beneficial.(16)
Other prophylactic therapies using methenamine, cranberry juice and probiotics have been evaluated in comprehensive studies and found to have insufficient evidence to support the use of such agents as prevention.(17-19)
TAKE HOME MESSAGES
-
The majority of febrile UTIs in infants and young children are upper UTIs, which carry a 10%–40% risk of renal scarring.
-
Upper UTIs predominantly present with high fever, with or without other symptoms such as vomiting and irritability, whereas lower UTIs usually present with urinary symptoms such as dysuria, urgency and frequency in an otherwise afebrile, well child.
-
Due to the non-specificity of symptoms of UTI in young children, screening for UTI with urine investigations should be done early, especially in the absence of an obvious source of infection.
-
Urine collected from a urine bag is a useful screening test for UTI in children who have not achieved urinary continence, although it is not a diagnostic test.
-
Urine culture is the gold standard for diagnosis of UTI. If the screening urine bag specimen is suggestive of UTI, urine culture needs to be obtained to confirm the diagnosis. A child who requires intravenous antibiotics should be referred to a tertiary hospital for collection of urine culture (through in-out catheterisation or clean catch).
-
If the child is assessed to be suitable for oral antibiotics, two separate midstream urine samples should be collected in the outpatient setting, with at least one sample collected prior to initiation of antibiotics.
-
Both upper and lower tract UTIs can be treated in the outpatient setting. Lower UTIs require one week of treatment, whereas upper UTIs require 14 days of antibiotics therapy. For upper UTIs, patients who are aged < 6 months, acutely unwell, unable to tolerate oral therapy or have complicated UTI will need inpatient parenteral antibiotic treatment.
-
Urine cultures should also be traced to ensure targeted antibiotic therapy, and all patients should be reviewed 48–72 hours after initiation of antibiotic therapy to evaluate their response to treatment.
-
Treatment of constipation with stool softeners is effective in preventing recurrent UTI in children without urinary tract abnormalities.
-
Indications for referral to a tertiary hospital are patients who require intravenous antibiotics and those who need further workup. Patients with febrile UTI or recurrent cystitis should be referred for follow-up imaging 4–6 weeks after acute infection.
A urine bag was placed on Jane. After half an hour, a urine specimen was successfully collected. A dipstick test showed a leucocytes value of 4+ and was positive for nitrites. As Jane was haemodynamically stable, a decision was reached after discussion with her mother for her to undergo outpatient treatment with a course of antibiotics. Jane’s mother was instructed to obtain two separate clean-catch urine specimens, which were sent to the laboratory for urinalysis and urine culture. Oral cephalexin was prescribed for 14 days with instructions to start antibiotics only after urine specimens were collected. A follow-up appointment was scheduled for Jane to be reviewed in two days. Urinalysis showed that her urine was pyuric and nitrites were positive. Her urine cultures revealed Escherichia coli with significant growth of > 100,000 cfu/mL, sensitive to cephalexin. On subsequent review, Jane’s fever had subsided after 48 hours of antibiotics, and she was back to her usual self. Her mother was advised to complete the whole course of antibiotics. A referral to a paediatric tertiary hospital was made for further investigations and follow-up.
SMJ-62-332.pdf


