Abstract
INTRODUCTION
Chest radiographs (CXRs) are widely used for the screening and management of COVID-19. This article describes the radiographic features of COVID-19 based on an initial national cohort of patients.
METHODS
This is a retrospective review of swab-positive patients with COVID-19 who were admitted to four different hospitals in Singapore between 22 January and 9 March 2020. Initial and follow-up CXRs were reviewed by three experienced radiologists to identify the predominant pattern and distribution of lung parenchymal abnormalities.
RESULTS
In total, 347 CXRs of 96 patients were reviewed. Initial CXRs were abnormal in 41 (42.7%) out of 96 patients. The mean time from onset of symptoms to CXR abnormality was 5.3 ± 4.7 days. The predominant pattern of lung abnormality was ground-glass opacity on initial CXRs (51.2%) and consolidation on follow-up CXRs (51.0%). Multifocal bilateral abnormalities in mixed central and peripheral distribution were observed in 63.4% and 59.2% of abnormal initial and follow-up CXRs, respectively. The lower zones were involved in 90.2% of initial CXRs and 93.9% of follow-up CXRs.
CONCLUSION
In a cohort of swab-positive patients, including those identified from contact tracing, we found a lower incidence of CXR abnormalities than was previously reported. The most common pattern was ground-glass opacity or consolidation, but mixed central and peripheral involvement was more common than peripheral involvement alone.
INTRODUCTION
Coronaviruses are enveloped positive-sense RNA viruses known to cause upper and lower respiratory tract infections in addition to gastrointestinal, hepatic and neurologic diseases.(1-3) While most infections are mild, coronaviruses have emerged as important pathogens since the severe acute respiratory syndrome (SARS) epidemic in 2002–2003. SARS was the first known coronavirus to cause severe respiratory illness in immunocompetent adults and has an overall mortality rate of 9.6%.(4) It predominantly affected elderly individuals aged above 60 years, with an estimated mortality rate as high as 43%–50% in this age group.(3) In 2012, the Middle East respiratory syndrome (MERS) emerged in Saudi Arabia, with an even higher mortality rate of 37.1%.(5)
In December 2019, a team from the Chinese Center for Disease Control and Prevention investigated an emerging cluster of patients with pneumonia of unknown aetiology in the city of Wuhan, Hubei Province, China.(6) The team subsequently isolated a novel coronavirus on 7 January 2020, which was later named as SARS-CoV-2.(7,8) This new virus represents the third virus of the Coronaviridae family to cause severe respiratory disease in the healthy population. Since then, the virus has spread worldwide, and the World Health Organization (WHO) declared the outbreak to be a public health emergency of international concern on 30 January 2020. WHO subsequently named the disease ‘coronavirus disease 2019’ (COVID-19) on 11 February 2020. As the number of confirmed cases increased to more than 118,000 with more than 4,200 deaths worldwide, WHO declared the outbreak a pandemic on 11 March 2020.(9)
Although chest radiography (CXR) is less sensitive than computed tomography (CT) in detecting ground-glass opacities,(10) it remains an essential initial radiological investigation in the screening and management of COVID-19 pneumonia. In Singapore, reverse-transcription polymerase chain reaction (RT-PCR) of respiratory samples is used as the standard for confirmatory diagnosis of COVID-19 infection. CXR is used for the initial detection of radiographic apparent pneumonia and triaging of suspected cases owing to its easy availability, instant diagnostic capability and low cost. Moreover, radiographic resolution of abnormalities has been used as an important criterion for discharging patients from the hospital, among other clinical parameters.(11) CXR promptly detects cases that are complicated by acute respiratory distress syndrome (ARDS), which warrants intensive care, including mechanical ventilation. Deterioration to ARDS generally indicates a poor prognosis – Chen et al found that 11 (64.7%) out of 17 patients with ARDS died of multiorgan failure,(12) while Zhu et al described a case of ARDS that showed worsening of diffuse consolidation on CXRs performed on Day 8 and Day 11 of symptoms, and the patient passed away on Day 21 despite being managed in the intensive care unit.(6) Finally, CXR plays a crucial role in excluding other causes of hypoxia that are imminently treatable. For example, although not encountered in our series and considered a rare complication, a case of pneumothorax was reported by Chen et al on initial CXR.(12)
Despite the widespread use of CXR for managing COVID-19, a limited number of studies have analysed the imaging features of this imaging modality. In this article, we described the radiographic findings of COVID-19 pneumonia across a spectrum of swab-positive patients. In our discussion, we contrasted our findings for COVID-19 with the reported features of other viral pneumonia, with attention to SARS and MERS, the two other lethal human coronavirus infections.
METHODS
For this retrospective review study, the requirement of obtaining informed consent from the study participants was waived by the Singapore Ministry of Health under the Infectious Diseases Act (Chapter 137). 96 patients who were confirmed as having COVID-19 and were admitted to four institutions in Singapore between 22 January and 9 March 2020 were included in this study. COVID-19 infection was confirmed by RT-PCR assay on two nasopharyngeal and throat swabs 24 hours apart at the National Public Health Laboratory. As little was known about the virulence of the pathogen at the onset of the pandemic, all patients were managed in negative pressure isolation rooms – 91 patients at the National Centre for Infectious Diseases, three at Singapore General Hospital, one at Changi General Hospital and one at Sengkang General Hospital. Patient demographics and clinical data were collected from electronic medical records.
Radiographic examinations were conducted using digital radiography equipment (Fluorospot Compact FD; Siemens, Erlangen, Germany, and FDR Visionary Suite and FDR Go; Fujifilm, Tokyo, Japan). All radiographs were acquired using frontal projections (posteroanterior views for patients who were able to stand, and anteroposterior views for portable studies and for patients who could not stand).
Initial and follow-up frontal CXRs of patients with confirmed COVID-19 were included for review. The DICOM (digital imaging and communications in medicine) images were anonymised and reviewed by three fellowship/subspeciality-trained thoracic radiologists with 12, 15 and 17 years of experience, respectively, using a 2,048 × 2,048 pixel monitor (Barco, Sunnyvale, CA, USA). For patients who underwent multiple follow-up CXRs, the radiographs were systematically reviewed by the three readers to select the radiograph deemed to have the worst findings for analysis. These radiographs were reviewed independently and the final decision was reached by consensus. The reviewers were blinded to the clinical findings of the patients.
Radiographs were assessed for the presence, predominant pattern and distribution of lung parenchymal abnormalities. The CXRs were analysed and categorised as either normal or abnormal. If abnormal, the dominant pattern of lung parenchymal abnormality was assessed; the extent of involvement was recorded as unifocal, unilateral multifocal or bilateral multifocal; and the distribution was then categorised as predominant central, peripheral or mixed.
Each lung field was divided into three zones: upper, middle and lower, with each zone spanning one-third of the craniocaudal distance of the lung field. An opacity was considered central if most of the abnormality was located within the medial two-thirds of the lung, and peripheral if most of it was located within the lateral one-third of the lung. The patterns of the lung parenchymal abnormalities were categorised as: consolidation (homogeneous opacification of parenchyma with obscuration of vessels); ground-glass (hazy opacification without obscuring vessels); nodular opacities (focal round opacities); or reticular opacities (linear opacities that form a mesh-like pattern). We also recorded the presence of any other associated findings such as pleural effusion, cavitation, lymphadenopathy and pneumothorax.
RESULTS
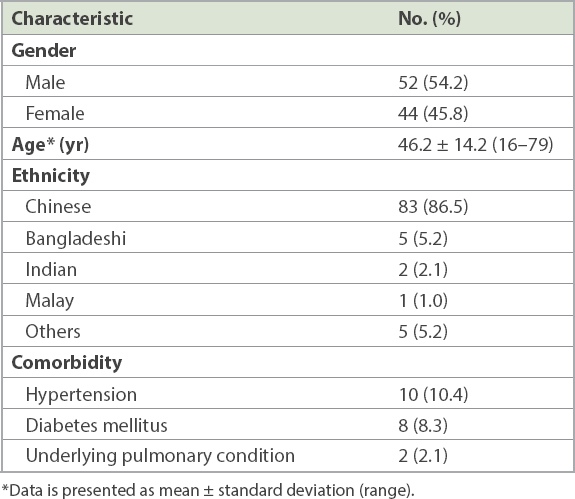
The study group consisted of 52 men and 44 women (mean age 46.2 ± 14.2 [range 16–79] years;
Table I
Summary of patient characteristics (n = 96).
In total, we reviewed 347 CXRs performed on 96 patients (mean CXR 3.6 ± 3.9; range 1–32 per patient). The mean duration from initial CXR to the follow-up CXR that was deemed to have the worst findings was 8.2 ± 9.8 days. Only one patient who required mechanical ventilation developed superimposed Klebsiella pneumonia. The mean duration from onset of symptoms for the 55 patients who presented with normal CXR at presentation was 5.9 ± 7.5 days. Of these 55 patients, 16 (29.1%) did not require follow-up CXRs because of clinical improvement. Of the remaining 39 (70.9%) patients, 13 (33.3%) subsequently showed abnormal CXR findings, with a slight majority presenting predominantly with ground-glass opacities.
At the initial presentation, 41 (42.7%) of the 96 patients had abnormal CXR (
Table II
Abnormal radiographic findings of COVID-19 pneumonia at initial presentation and follow-up.
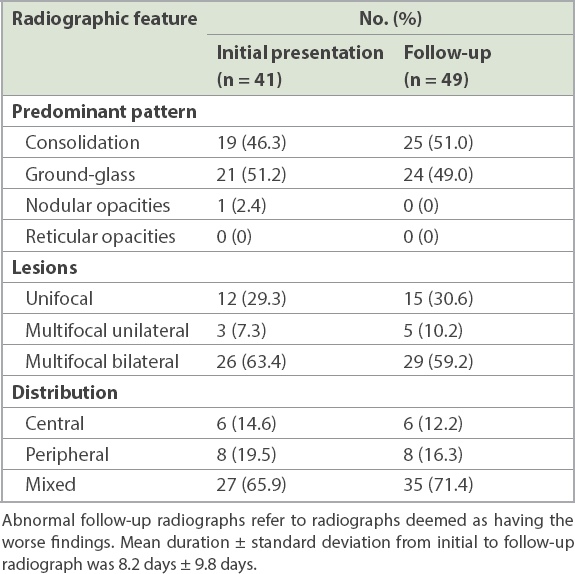
Fig. 1
A 35-year-old man with fever and cough for one day. Chest radiograph obtained on Day 1 of symptoms shows a unifocal consolidation in the right lower zone, predominantly in the infrahilar location.
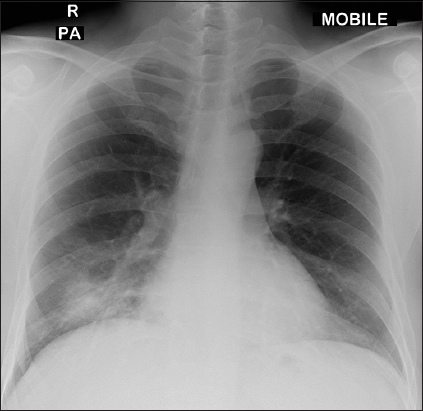
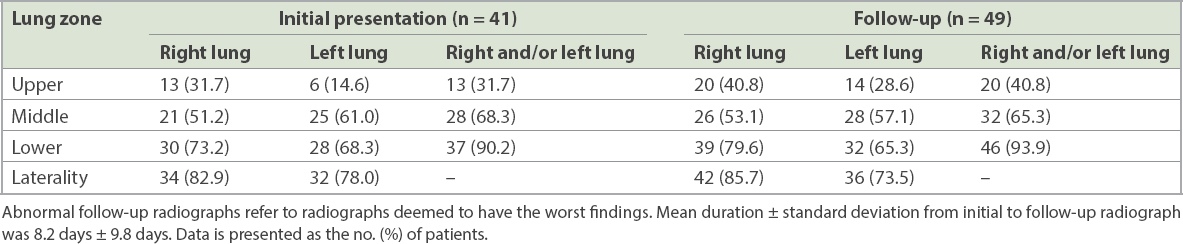
Table III
Location of lung parenchymal abnormalities in COVID-19 pneumonia on initial and follow-up chest radiographs.
In follow-up radiographs that were deemed abnormal (n = 49), consolidation was the predominant pattern, as observed in 25 (51.0%) of the 49 patients (
Fig. 2
A 55-year-old woman with fever, cough and dyspnoea for four days. (a) Initial chest radiograph obtained on Day 4 of symptoms shows multifocal ground-glass opacities in the bilateral lower zones (arrowheads). (b) Follow-up radiograph performed two days later shows increased density in the left lower zone (white arrowheads), in keeping with consolidation. A small focus of consolidation is also seen in the right lower zone (black arrowhead).
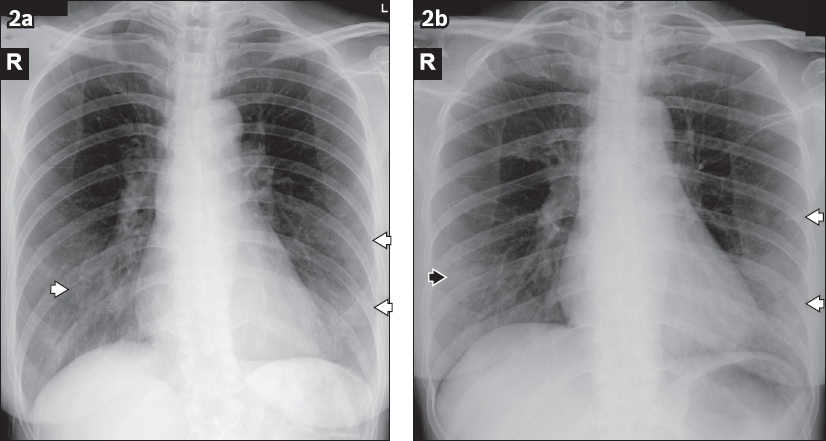
Fig. 3
A 77-year-old woman with fever, cough and sore throat for five days. (a) Initial chest radiograph on admission is normal. (b) Follow-up radiograph performed two days later shows interval development of multifocal ground-glass opacities in the bilateral lower zones (arrowheads). The patient deteriorated during admission and required intensive care admission for mechanical ventilation. (c) Radiograph obtained on Day 11 of admission shows progression of lung changes with multifocal bilateral lung consolidation, worse in the left lung.
DISCUSSION
In many parts of the world, CXRs are commonplace in the management of COVID-19. Better understanding of CXR features in this disease would enable appropriate triaging and treatment evaluation. At present, publications on chest CT in COVID-19 outnumber those on CXRs.
From our experience, the advantage of detecting subtle pulmonary involvement that is not depicted on CXR can be outweighed by the logistical challenges of transferring patients out of their isolation rooms into the radiology department for CT and the potential risk of disease transmission to healthcare workers and other patients. Further, we have observed that subsequent serial radiographs help to detect the progression of pulmonary involvement without compromising patient care. In a retrospective review of 21 patients by Chung et al, 18 (85.7%) patients showed abnormalities on initial chest CT images.(13) In a larger study of 138 patients, all patients showed positive chest CT findings with bilateral distribution of lung parenchymal abnormalities.(14) Chest CT may be used in a setting where urgent detection and diagnosis of COVID-19 infection is required while awaiting results from respiratory samples, although this would depend on the pre-test probability of COVID-19. For these reasons, we do not recommend CT as an initial diagnostic test. This is in line with the recent recommendations by the American College of Radiology and the British Society of Thoracic Imaging, which state that CT should be utilised for specific clinical indications, and should not be used to screen for COVID-19 or as a first-line test to diagnose COVID-19.(15,16) A recently published consensus statement from the Fleischner Society recommends imaging for patients with mild symptoms who are at risk of disease progression, patients with moderate to severe features, and those with worsening respiratory status.(17) We have, therefore, sought to methodically study the CXR findings in our initial national cohort of patients.
In this retrospective study of the initial 96 confirmed cases of COVID-19 in Singapore, 55 (57.3%) patients had normal initial CXR. This proportion is higher than that in Wong et al’s study, where 31% of the 64 patients had normal initial CXR,(18) and similar to that quoted in the review by Hosseiny et al.(19) This discrepancy could be attributed to aggressive contact tracing as a disease containment strategy in Singapore, resulting in early detection and isolation of symptomatic patients with COVID-19. It is also lower than the reported incidence of abnormal CT findings.(20) Still, it is not unexpected, given the higher sensitivity of CT for lung disease, particularly the ground-glass changes that predominate in COVID-19. We believe that our reported incidence rate of abnormal CXR better reflects the spectrum of COVID-19 infections.
In our series, the middle and lower lung zones were predominantly involved, alluding to dominant lower lobe disease. These findings concur with recent publications on CT findings of COVID-19 pneumonia, which showed that multifocal bilateral lung involvement – worse in the lower lobes – was the predominant abnormality.(13,21-24) Similarly, a preliminary study on the ultrasonography findings of COVID-19 pneumonia found that the posterior lungs, particularly in the lower lung fields, were commonly involved.(25)
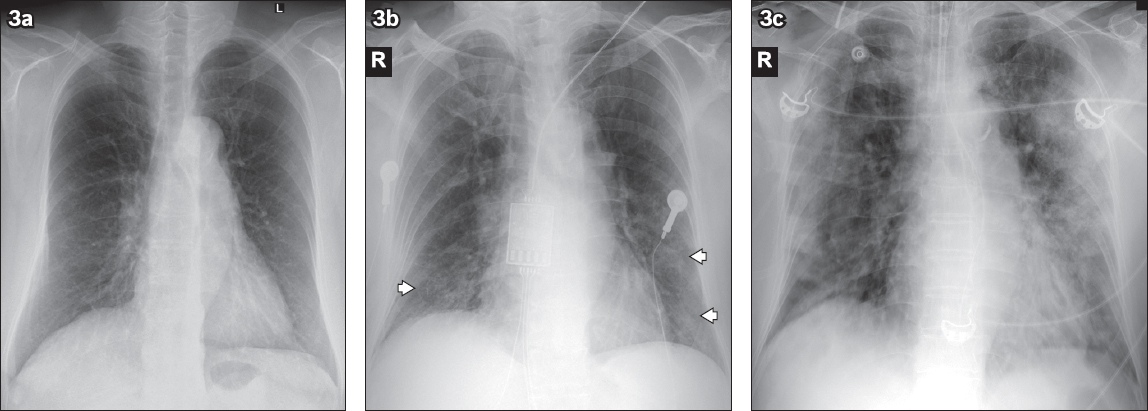
In our analysis, combined central and peripheral involvement was more common, unlike recent publications on COVID-19, which reported that peripheral distribution was more commonly observed on CT(13,21-24) and CXR.(18,19) A plausible explanation for the greater central radiographic distribution of consolidation in our series is the summation effect of the abnormalities that are located posteriorly and medially in the lower lobes on 2D analysis, which, although peripheral in distribution on CT, may be projected as central (i.e. close to the hilum on CXR), as demonstrated in
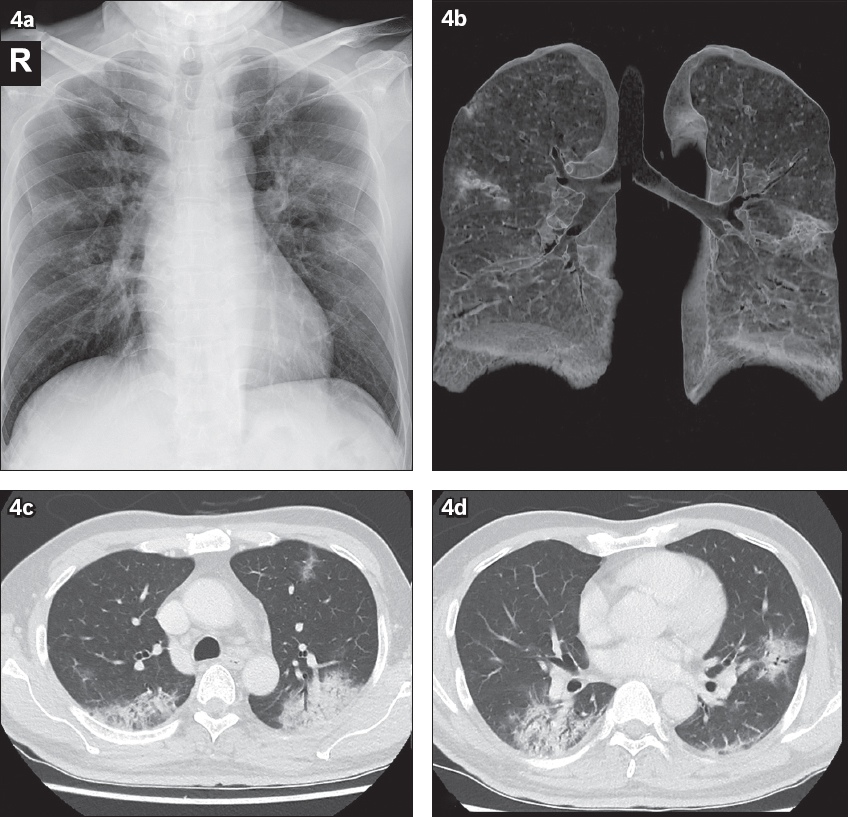
Fig. 4
A 56-year-old man with a travel history to China who presented with cough and sputum production. Imaging studies were all performed on Day 12 after the onset of symptoms. (a) Chest radiograph shows multifocal bilateral lung consolidation, involving all three zones of the right lung as well as the left upper and middle zones. (b) Volume-rendered maximum intensity projection 3D CT image shows the pattern of lung involvement, which is predominantly peripheral in location, involving the posterior and dependent areas of the lungs. (c & d) Axial contrast-enhanced CT images in lung window show multifocal confluent areas of consolidation that are extended to the subpleural and peripheral locations. Note the patchy scattered pure ground-glass opacities, and the absence of nodules and reticular opacities; these were not evident on chest radiograph. There is an absence of both thoracic nodal enlargement and pleural effusion.
Based on our findings, the predominant pattern of lung parenchymal abnormality was that of ground-glass opacity (51.2%) on initial presentation. This is in line with the published literature, which reported ground-glass opacities, mixed ground-glass opacities and consolidation as the predominant lung parenchymal abnormalities on CT.(13,21-24) Our results showed an almost equal proportion of ground-glass opacity and consolidation in both initial and follow-up radiographs, with a slight predominance for ground-glass opacity. It is known that CT has higher sensitivity for detecting mild ground-glass opacities compared with CXR. However, it is not known whether the detection of such mild ground-glass opacities necessarily portends a poorer prognosis. By contrast, patients with more severe disease requiring extended hospitalisation have been found to have a greater prevalence of consolidation, which can be readily appreciated on CXR.(28)
The imaging features of COVID-19 have been compared with those of SARS and MERS. The review by Hosseiny et al has highlighted the overlapping radiographic features of the three human coronavirus infections, but this was based on extrapolation of reports from CT images of COVID-19.(19) Nonetheless, our study confirms that ground-glass opacity and consolidation predominate in the lower lobe and posterior lungs in COVID-19.
In a retrospective review of 138 patients with SARS in Hong Kong, Wong et al reported that 78.3% of the patients had airspace opacities with ill-defined margins, which obscured the vasculature compatible with consolidation. The involved lung zones were predominantly middle (52.8%) and lower (64.8%), with peripheral location (75%) on initial radiographs.(29) Our findings showed a slight predominance of ground-glass opacity on initial radiographs, and this was the most common parenchymal abnormality in a predominantly mid- and lower-zone distribution. However, unifocal distribution was more common in SARS,(29,30) in contrast to multifocal bilateral lung involvement observed in 63.4% of our patients. We illustrate this difference with two cases of patients from our local institution who were diagnosed with SARS in 2003; one of them developed multifocal peripheral consolidation, while the other had unifocal consolidation (
Fig. 5
(a & b) A 72-year-old woman was diagnosed with SARS in 2003. (a) Initial chest radiograph obtained on Day 5 of symptoms shows bilateral lung consolidation in the right middle as well as left middle and lower zones, which appear predominantly peripheral in location. (b) Follow-up radiograph performed 13 days later shows worsening lung changes with diffuse bilateral lung consolidation, in keeping with the picture of acute respiratory distress syndrome. The patient succumbed to the disease one day later despite intensive care support. (c) A 68-year-old man with SARS was admitted in 2003. Chest radiograph obtained on Day 6 of symptoms shows a focus of unifocal ill-defined consolidation with concomitant reticular markings in the right middle zone. The patient was discharged well after two weeks.
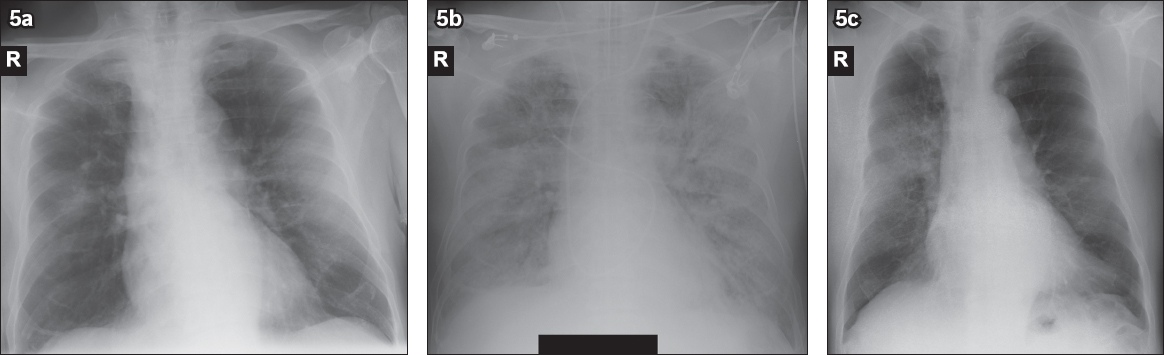
Fig. 6
Chest radiograph illustrations show the typical lung parenchymal abnormality and distribution in (a) COVID-19 pneumonia – multifocal bilateral lung ground-glass opacities in mixed central and peripheral distribution; (b) SARS – unifocal right lung consolidation, predominantly in peripheral distribution; and (c) MERS – unilateral ground-glass opacity in the peripheral location.
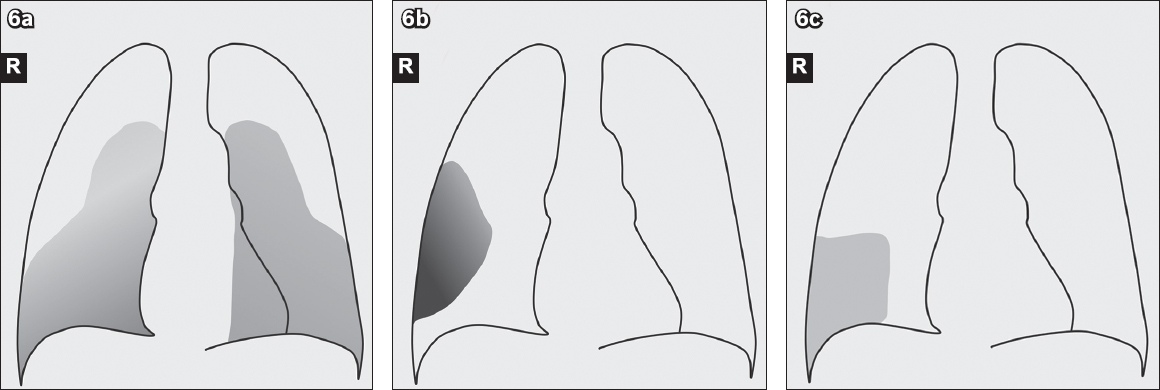
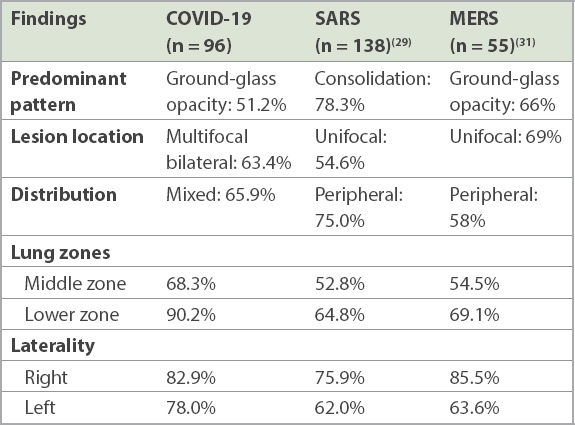
Table IV
Predominant initial radiographic findings in coronavirus disease 2019 (COVID-19) pneumonia, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS).
Chung et al reported that ground-glass opacities were more common on CT, as observed in 12 (57%) out of 21 patients with COVID-19 pneumonia, while consolidation with ground-glass opacities was observed in 6 (29%) out of 21 patients.(13) In addition, a peripheral location of opacities was common (33%).(13) Both Chung et al and Huang et al reported that multifocal bilateral lung involvement was a commonly observed pattern.(11,13) Ground-glass opacities and consolidation in subpleural regions were the predominant early CT findings in SARS, while extensive ground-glass opacities and consolidation in basilar and subpleural distributions were common in MERS.(26,27) On CT, multifocal bilateral involvement has been more commonly observed in COVID-19 pneumonia and MERS, as opposed to unifocal involvement in SARS,(11,13,26,27) although this may be confounded by the more widespread use of CT in the current pandemic.
Viral pneumonia has a variable appearance on CXR and CT. Moreover, co-infection with a bacterial pathogen or inflammatory lung disease often confounds radiologic findings. Nevertheless, most patterns of viral pneumonia exhibit similarities on the basis of viral families or viridae, as they share similar pathogenic mechanisms.(3) The finding of multifocal bilateral lung consolidation may overlap with other infective pneumonia and may, therefore, be difficult to distinguish on imaging. Viral pneumonias of adenovirus, herpes simplex virus and bocavirus aetiology are known to cause multifocal or diffuse bilateral lung consolidation, while cytomegalovirus pneumonia may show bilateral ground-glass opacities and airspace consolidation.(3) On CXR, influenza pneumonia typically demonstrates as bilateral reticulonodular opacities with or without consolidation and predominantly involves the lower lobes; on CT, centrilobular nodules are commonly observed in peripheral distribution, although ground-glass opacities and consolidation have also been described.(3,32,33) Bacterial pneumonias mainly show lobar or broncho-pneumonia and present as extensive patchy consolidations of lung parenchyma on CT, while mycoplasma pneumonia manifests as bronchial wall-thickening and centrilobular nodules.(34)
SARS-CoV-2 has been reported to invade the bronchioles, causing bronchiolitis and peribronchitis with subsequent distal spread, thus giving rise to initial ground-glass opacity and subsequent lobular patchy opacity when the entire secondary pulmonary lobule is involved.(35) In addition, further involvement of the interlobular interstitium results in the ‘fine reticulation’ sign.(35) Variable findings of COVID-19 pneumonia, depending on the time course of the disease at which the imaging is performed, may pose a diagnostic challenge to radiologists. Ground-glass opacities observed on initial CT were shown to progress to consolidation during the peak stage of the disease (9–13 days from onset of symptoms) before showing interval improvement after 14 days.(36) In our study, the mean duration from onset of symptoms to an abnormal CXR finding was 5.3 ± 4.7 days. Hence, the interpretation of radiographic and CT findings in a suspected or confirmed case should be correlated with the duration of symptom onset to improve diagnostic performance.
This retrospective study is limited by a lack of correlation with patient symptoms and long-term outcome. Nevertheless, our observations of certain predominant radiographic patterns are informative in dealing with this novel respiratory tract infection, and we have also highlighted specific key differences in findings from recent publications on CXR features of COVID-19, which have been mainly limited to describing chest CT. As the current pandemic continues to evolve, we expect to better determine the natural progression of radiographic findings in relation to novel treatment regimens such as the use of antiretroviral medications (e.g. combination of lopinavir and ritonavir).(11)
In conclusion, CXR is widely used as a tool for prompt initial evaluation and subsequent management of COVID-19 pneumonia. However, its imaging features have not been as well-described as those for chest CT. We found the incidence of abnormal CXR on presentation to be lower, and mixed central and peripheral involvement to be more common than previously reported. Improved understanding of the patterns of radiographic findings will enhance diagnostic workflow and the appropriate use of CXR in public health measures to deal with the current pandemic.
About the First Author

Dr Khoo Hau Wei is an Associate Consultant at the Department of Diagnostic Radiology, Tan Tock Seng Hospital, Singapore, with a subspecialty interest in head and neck imaging. He is a Fellow of the Royal College of Radiologists in the United Kingdom. During his residency training in the National Healthcare Group Diagnostic Radiology Residency Programme, he had the opportunity to participate in several research projects relating to COVID-19 under the leadership of A/Prof Tan Cher Heng and Dr Gregory Kaw. Moving forward, he would like to further deepen his research in head and neck imaging and contribute to medical education.
ACKNOWLEDGEMENT
Recruitment of study participants and sample collection were funded by a seed grant from the Singapore National Medical Research Council (TR19NMR119SD; project reference number: CCGSFPOR20001).


