Abstract
INTRODUCTION
This retrospective review aimed to examine the relationship between preoperative pulmonary function and the Cobb angle, location of apical vertebrae and age in adolescent idiopathic scoliosis (AIS). To our knowledge, there have been no detailed analyses of preoperative pulmonary function in relation to these three factors in AIS.
METHODS
A total of 38 patients with thoracic or thoracolumbar scoliosis were included. Curvature of spinal deformity was measured using the Cobb method. Forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) were used to evaluate preoperative pulmonary function. Statistical methods were used to analyse the relationship between preoperative pulmonary function and the factors that may contribute to poor pulmonary function.
RESULTS
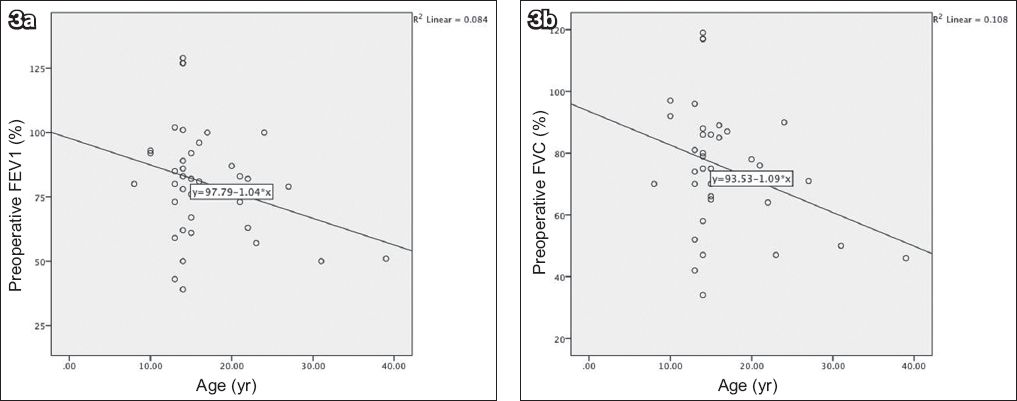
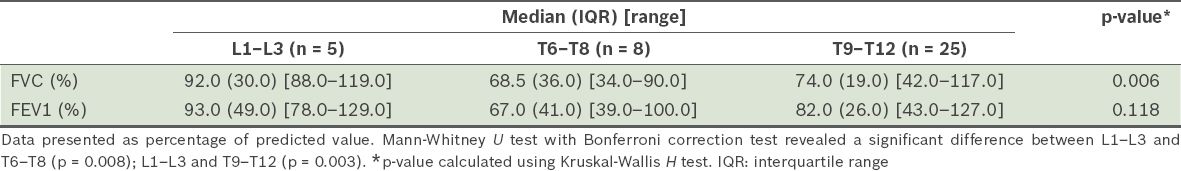
The mean age of the patients was 16.68 ± 6.04 years. An inverse relationship was found between the degree of the Cobb angle and FVC as well as FEV1; however, the relationships were not statistically significant (p = 0.057 and p = 0.072, respectively). There was also a trend towards a significant negative correlation between the thoracic curve and FVC (p = 0.014). Patients with larger thoracic curves had lower pulmonary function. A one-year increase in age significantly decreased FVC by 1.092 units (p = 0.044). No significant relationship between age and preoperative FEV1 was found. The median FVC was significantly higher in patients with affected apical vertebrae located at levels L1–L3 than at T6–T8 or T9–T12 (p = 0.006).
CONCLUSION
Lung function impairment was seen in more severe spinal deformities, proximally-located curvature and older patients.
INTRODUCTION
Scoliosis is a three-dimensional deformative abnormality of the spine. Approximately 85% of cases are idiopathic.(1,2) Based on the age of presentation, scoliosis is further categorised as infantile, juvenile or adolescent idiopathic. Adolescent idiopathic scoliosis (AIS), which accounts for the majority of the three categories, presents at age ten and lasts till the end of growth.(3) Its prevalence is dependent on the curvature of the spine and gender of the patient, and is higher among females, who have been observed to have more severe curvature.(4)
There is substantial interest in the relationship between spinal deformity and pulmonary function due to the potentially high rates of morbidity and mortality when progressive scoliosis results in pulmonary impairment. Decreasing pulmonary function is a major concern in progressive severe scoliosis. Once documented, the progression of scoliosis needs to be addressed to arrest thoracic cage deformity and concomitant pulmonary compromise.(5) Thoracic cage deformity can arise intrinsically from fused ribs and/or secondarily from the curvature, rotation and shortening of the thoracic spine. Severe thoracic cage distortion leads to extrinsic restrictive lung disease from the diminution of lung volume under the convex rib hump and on the concave side, where the ribs impinge on the lung.(6) Thoracic cage deformity often accompanies spine deformity in patients with AIS. The deformed structures compress the lung parenchyma, causing a decrease in lung volume and compliance. These changes, along with the increased effort to breathe, may result in alveolar hypoventilation, hypercapnia and hypoxaemia. Due to hypoxaemia and vascular bed restriction, pulmonary hypertension follows, leading to cor pulmonale and right-sided heart failure.(7)
Previous studies have indicated that severe scoliosis leads to poor pulmonary function.(2-5,8-17) Since poor pulmonary function may lead to a higher incidence of postoperative pulmonary complications, preoperative pulmonary function tests (PFTs) have commonly been used to predict postoperative pulmonary complications.(14) However, there have been no detailed analyses of preoperative pulmonary function in relation to the Cobb angle, location of apical vertebrae and age in AIS. Hence, the current review was conducted to examine the relationship between preoperative pulmonary function and these three factors among patients with AIS.
METHODS
A retrospective record review was conducted among 38 AIS patients with thoracic involvement (thoracic or thoracolumbar scoliotic curvature) who were admitted to our institution for surgical intervention between July 2000 and December 2013. The patients enrolled in the study were (a) aged 13–24 years, (b) had a preoperative diagnosis of AIS and (c) had preoperative lung function tests available for analysis. The spinal curve angle of all the patients was measured using the Cobb angle on anterior-posterior radiographs based on the standard measurement technique.(4,18,19) Three measurements were performed and their average was used for analysis. Surgical correction of the spine was indicated when there was a Cobb angle of greater than 45º or a progressive Cobb angle of more than 5º at three- and six-month follow-up (
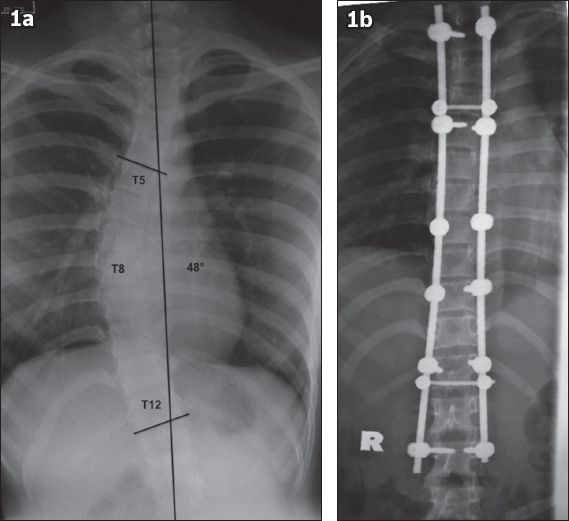
Fig. 1
Posterior-anterior plain radiographs of a 23-year-old woman show right thoracic idiopathic scoliosis of 48º (a) before surgery and (b) after surgery. T5: upper end vertebra; T8: apical vertebra; T12: lower end vertebra
All patients underwent standard PFTs prior to surgery using a SpiroUSB™ Spirometer ML2525 (CareFusion, CA, USA). PFTs were used to measure total lung capacity (TLC), forced vital capacity (FVC) and forced expiratory volume in one second (FEV1). Each test was repeated three times and the single best effort was recorded. FVC gives an assessment of lung volume, while the FEV1 provides an assessment of flow function. Age, gender and height-matched standards published by the American Thoracic Society(20) were used to generate a percentage of the predicted value for each pulmonary parameter. Pulmonary function was considered normal when TLC, FVC and FEV1 were > 80% of the predicted values. Mild pulmonary impairment was defined as TLC, FVC and FEV1 ≤ 80% but > 65% of the predicted values; moderate pulmonary impairment was defined as TLC, FVC and FEV1 ≤ 65% but ≥ 50% of the predicted values; and severe pulmonary impairment was defined as TLC, FVC and FEV1 < 50% of the predicted values.(5)
Simple linear regression was performed to analyse the relationship between the Cobb angle and preoperative pulmonary function. The Kruskal-Wallis H test and Mann-Whitney U test were used to compare lung function according to the different levels of affected spinal vertebrae. Data entry and analysis were performed using IBM SPSS Statistics version 18.0 (IBM Corp, Armonk, NY, USA).
RESULTS
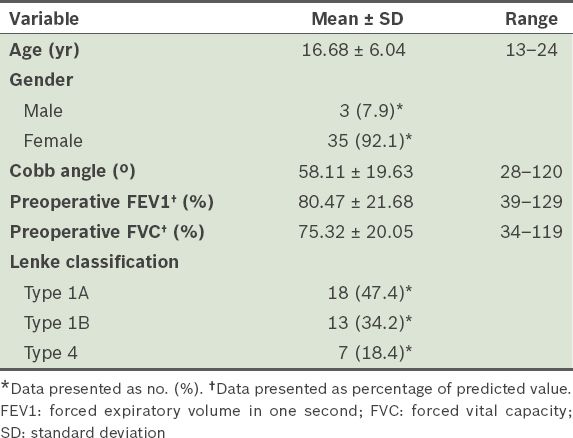
A total of 38 patients were included; their mean age was 16.68 ± 6.04 years. A majority of the patients were female (92.1%). The mean Cobb angle was 58.11º ± 19.63º. The mean preoperative FVC and FEV1 were 75.32% ± 20.05% and 80.47% ± 21.68%, respectively. The majority of patients had Type 1A (47.4%) and Type 1B (34.2%) scoliosis, according to the Lenke classification (
Table I
Demographic characteristics of the patients with adolescent idiopathic scoliosis (n = 38).
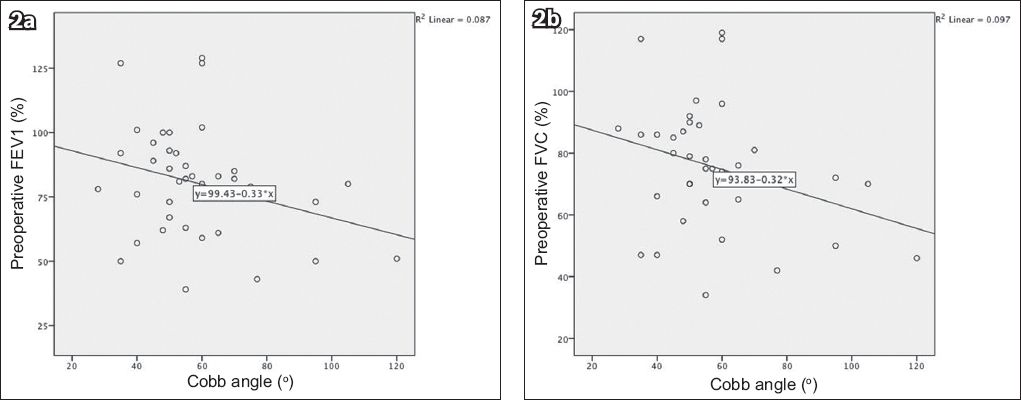
The relationships between the Cobb angle and preoperative FVC and FEV1 are shown in
Table II
Relationships between age, Cobb angle and preoperative lung function indices (FVC and FEV1).
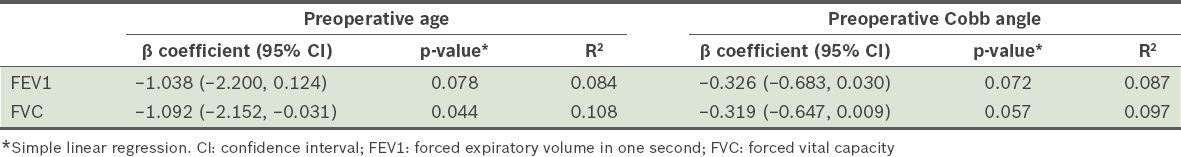
Fig. 2
Scatter charts show the relationship between the Cobb angle and (a) preoperative forced expiratory volume in one second (FEV1) and (b) preoperative forced vital capacity (FVC).
A one-year increase in age significantly decreased FVC by 1.092 units, as shown in
Fig. 3
Scatter charts show the relationship between age and (a) preoperative forced expiratory volume in one second (FEV1) and (b) preoperative forced vital capacity (FVC).
The median FVC was significantly higher in those with affected apical vertebrae at L1–L3 (92.0%, interquartile range [IQR] 30.0%) than in those with affected apical vertebrae at T6–T8 (68.5%, IQR 36.0%; p = 0.008), and at L1–L3 (92.0%; IQR 30.0%) than in those with affected apical vertebrae at T9–T12 (74.0%, IQR 19.0%; p = 0.003) (
Table III
Median forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) according to levels of apical vertebrae.
No significant difference was found in the median FVC and FEV1 at the different levels of upper end vertebrae (
Table IV
Median forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) according to levels of upper end vertebrae.
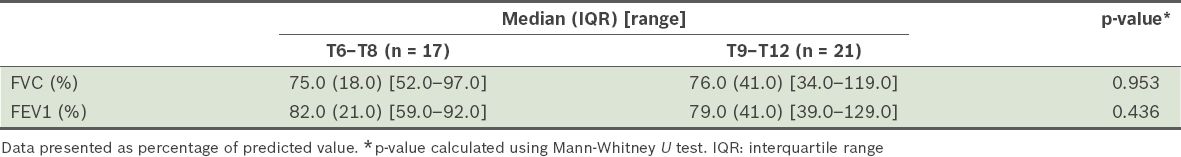
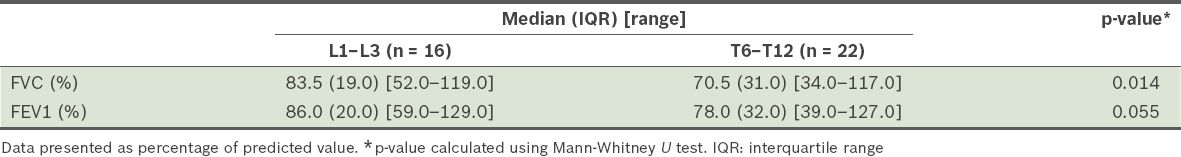
Table V
Median forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) according to levels of lower end vertebrae.
DISCUSSION
AIS affects pulmonary function in a variety of ways. Apart from decreasing the volume and compliance of the lung parenchyma as mentioned earlier, the component of vertebral rotational deformity in the transversal plane leads to asymmetrical deformity of the thorax, which some consider to be the fourth dimension of scoliosis.(21,22) A deformed thoracic cage increases the stiffness of the chest wall, reduces the force of the respiratory muscles and increases the mechanical dysfunction of the diaphragm.(15,17) These changes are more pronounced in early-onset scoliosis before the age of five years and in congenital types where vertebral anomalies and fused ribs are present. Therefore, the respiratory impairment observed in patients is restrictive in nature.(3,17)
The Cobb angle was used to measure the curvature of spinal deformity as it is practical and convenient.(5,18) Researchers have debated the accuracy of the correlation between the Cobb angle and respiratory function.(23-25) However, their concerns tend to be the curvature deformities in early-onset scoliosis, not AIS.(24) The current study showed a negative relationship between the degree of the Cobb angle and pulmonary function, although the relationship was not statistically significant. An inverse relationship between spinal deformity and pulmonary function was reported in previous studies. Vitale et al(6) found that the degree of the thoracic curve was negatively correlated with FEV1. There was also a trend toward a significant negative correlation between the thoracic curve and FVC.(6) Patients with a larger thoracic curve had lower pulmonary function measured by FEV1 and FVC.(6)
Pulmonary function is affected by the severity of the thoracic curve, number of vertebrae involved in the curvature, thoracic hypokyphosis and coronal imbalance. These four factors were associated with increased risk of moderate to severe pulmonary impairment. However, the effects were minimal. Only 19.7% of the observed variability in FVC was significantly impaired. Similar results were found for FEV1 (18.0%) and TLC (8.8%).(5) Respiratory failure was seen in untreated patients who had AIS with a large scoliotic angle and low vital capacity (VC), especially in those with early-onset curvature.(13) A scoliotic angle greater than 40º and kyphotic angle greater than 50º was correlated with increased pain and decreased FVC; reduction in FVC was also correlated with curve rigidity.(26)
Spinal deformity reduces the VC of the lungs, primarily by affecting the TLC. A large scoliotic angle is associated with reduced VC percentage. The cephalad location of the curvature, loss of normal thoracic kyphosis and a higher number of vertebrae involved also had an equal and additive influence on pulmonary impairment.(12) Aaro and Ohlund evaluated chest computed tomography images of 33 patients with AIS and reported reduced lung volumes; the effect on pulmonary function was greater in patients with coronal deformity than in those with sagittal deformity.(8) Increased frequency of respiratory impairment was also found in 50 patients aged 11–78 years with untreated idiopathic scoliosis, especially those with a severe degree of scoliosis.(27) Alternatively, Smyth et al found that the strength of respiratory muscles to achieve maximum respiratory pressure is a more important determinant of developing respiratory impairment than the degree of spinal curvature.(9) Impaired pulmonary function was also observed in patients with mild, idiopathic scoliosis. Muirhead and Conner concluded that the underlying impairment was restrictive in nature, as patients had a normal residual volume but reduced VC and TLC.(28) In early-onset curvature, reduction in VC was influenced by the degree of deformity. This had minimal or no effect on patients with onset in adolescence.
In the current study, patients with thoracolumbar curves were also included, in addition to those with thoracic scoliosis. Thoracolumbar curves may also distort the mechanics of the thorax as well as the functional efficacy of the diaphragm, thus affecting pulmonary function. Our study found that spinal curvature in a more cranial location resulted in greater impairment in the pulmonary function of patients. The findings were in agreement with results from other studies. Previous studies found that some patients with moderate curves were found to have clinically significant respiratory impairment during preoperative PFTs, attributed to factors such as curvature in a more cranial location.(5,12) A significant association between severity of kyphosis and respiratory impairment was also reported. Respiratory impairment was greater in a more cranially located kyphosis, especially above the T10 level.(29) Preoperative lung function was clinically impaired in 19% of patients with AIS, and this was significantly correlated with main thoracic and sagittal plane deformity severity, as well as proximal thoracic curve severity to a lesser degree.(17) In another study, Sakić et al examined different levels of apical vertebrae of the thoracic curve and pulmonary function.(11) Researchers categorised patients into two groups, upper thoracic scoliosis (apex vertebrae T5–T8) and lower thoracic vertebrae (apex vertebrae T9–T11). It was found that only upper thoracic scoliosis with a Cobb angle > 70º was correlated with restrictive ventilation disorder (68% VC) and latent hypoxemia demonstrated during the exercise tolerance test.(11) In contrast with the current finding, the degree of the curve in thoracolumbar scoliosis had no significant correlation with pulmonary function scores.
The categorisation of idiopathic scoliosis is made based on the age of first presentation, as it is difficult to determine the age of onset of the pathology.(30) Early-onset scoliosis, as in cases of infantile and juvenile scoliosis, is often associated with progressive deformation of the spine. Eventually, thoracic growth is affected. Respiratory morbidities and risk of thoracic insufficiency syndrome are more frequently reported than late-onset scoliosis. In the latter subtype, which includes AIS, clinically significant cardiorespiratory problems are observed in cases of severe curvature exceeding 60º.(10,31-33) The need for surgery should be considered when conservative treatment of AIS fails, or when there is evidence of curvature progression to a Cobb angle of 40º–50º or a large curve of 40º–50º.(33,34) Unlike AIS, surgical treatments for early-onset patients are mainly indicated early in the course of the disease to prevent highly predictable respiratory morbidity.(25) Another important factor shown in our results that may influence pulmonary function is the age of the AIS patient prior to surgery. As the patient gets older, pulmonary function worsens, as evidenced by the significant decline in FVC. This is supported by a previous study which suggested that ageing was a major factor of worsening ventilatory capacity of patients with AIS, leading to a higher risk of developing respiratory failure.(34) Pulmonary reserves peak in the second decade of life and gradually decline as the patient ages.(16,35,36)
Impairment in pulmonary function, which is expected with progression of scoliotic curvature, may be controlled with surgical correction. Therefore, pulmonary impairment from spinal deformity remains an indication for surgical intervention for AIS. Pulmonary function testing is useful in the preoperative evaluation of patients. Based on the results of the present study, impairment of function was seen in more severe cases of spinal deformity, proximally-located curvature and older patients.


