Dear Sir,
A 43-year-old Chinese woman, who had a history of Graves’ disease and a hemithyroidectomy five years before, presented to the emergency department with a week’s history of fever, productive cough, white sputum and palpitations. She was noted to be in fast atrial fibrillation and tachypnoeic. Upon consulting the endocrinology department, the clinical impression was of thyroid storm. The patient was promptly started on propylthiouracil, Lugol’s iodine, intravenous propranolol and intravenous hydrocortisone. She subsequently became hypotensive and unresponsive. She was quickly intubated and admitted to the intensive care unit with the preliminary diagnosis of thyroid storm secondary to sepsis, and received noradrenaline and dobutamine for inotropic support.
Urgent transthoracic echocardiography showed a ‘blob-like’ mass attached to the tip of the anterior mitral leaflet, which was suggestive of a vegetation or leaflet redundancy with moderate-to-severe mitral regurgitation. The ejection fraction was 72%. A diagnosis of mitral valve infective endocarditis was made. In view of the history of recent chest infection, the patient was empirically started on Tamiflu, moxifloxacin, vancomycin and ceftriaxone. Blood cultures were subsequently positive for Group B Streptococcus. Hence, the diagnosis was mitral valve infective endocarditis complicated by mitral regurgitation. The patient’s antibiotics were subsequently changed to benzylpenicillin and gentamicin. Initial management was geared towards treating the mitral regurgitation. However, the patient repeatedly failed extubation, with the presence of flash pulmonary oedema.
Serial transthoracic and transoesophageal echocardiography were performed (Figs.
Fig. 1
(a) Initial transthoracic echocardiogram shows infective endocarditis involving the mitral valve. (b) Transoesophageal echocardiogram shows the left ventricular pseudoaneurysm.
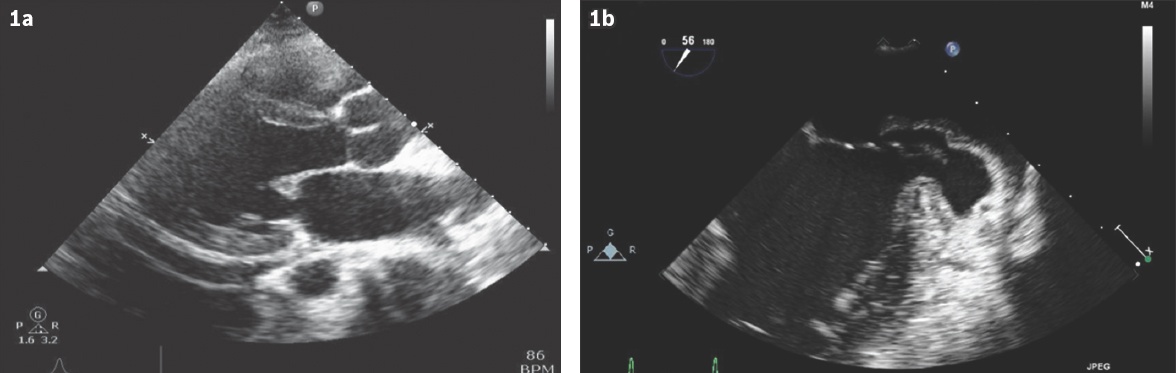
As the patient had multiple failed extubations due to the flash pulmonary oedema secondary to severe mitral regurgitation, urgent surgery was planned. Surgical risk of 30%–40% was estimated, including stroke and death on table. The patient underwent mitral valve replacement with left ventricular pseudoaneurysm repair and tricuspid valve repair. A vegetation was found on the posterior mitral leaflet, mainly in the C1 area, with a 3-cm gap between the atrium and ventricle leading into the left ventricular pseudoaneurysm. The ventricular pseudoaneurysm was debrided. The atrioventricular dissociation was repaired with bovine pericardial patches (Baxter Healthcare Corp, Santa Ana, CA, USA) on the atrial and ventricular side using 5-0 Prolene sutures in two layers, and the mitral valve was replaced on top of the patches using a Mosaic 25-mm bioprosthetic mitral valve (Medtronic, Minneapolis, MN, USA). Stable placement of the bioprosthetic mitral valve and exclusion of the pseudoaneurysm was confirmed on transoesophageal echocardiography. Tricuspid regurgitation was trivial.
The patient was extubated on the first postoperative day, then underwent intensive rehabilitation before being discharged. She completed six weeks of combination intravenous antibiotics during her stay in hospital. At one year post procedure, transthoracic echocardiography showed a stable bioprosthetic mitral valve with normal left heart function. She had resumed working and travelled overseas regularly.
Infective endocarditis is an endovascular microbial infection of intracardiac structures facing the blood, including the large intrathoracic vessels and intracardiac foreign bodies.(1) Local complications of infective endocarditis involve the valve or perivalvular region. Vegetations are usually attached to the atrial aspect of atrioventricular valves and the ventricular aspects of semilunar valves, at the valve line closure. Infection can spread to the aortic wall, which can cause sinus of Valsalva aneurysms, ring abscesses, tunnels, and fistulae of the cardiac chambers or the pericardial cavity, resulting in cardiac rupture and tamponade. Transoesophageal echocardiography can detect complications such as paravalvular abscesses or mycotic aneurysms. Involvement of the atrioventricular conduction system can cause an atrioventricular block, while rupture of the membranous septum results in ventricular septal defect.
Left ventricular aneurysm is very uncommon in infective endocarditis, usually presenting in cases of myocardial infarction.(2) Left ventricular aneurysm in a case of endocarditis has been described following aortic valve replacement.(3) In our case, transoesophageal echocardiography was crucial to the patient’s preoperative assessment and illustrated the anatomy well, as seen in
This case illustrated the successful repair of left ventricular pseudoaneurysm with bovine pericardial patches (Baxter Healthcare Corp) used to buttress the atrioventricular dissociation and restore atrioventricular continuity, and stable result obtained up to one year post procedure. To our knowledge, this is the first report of the formation of left ventricular pseudoaneurysm from Group B Streptococcus infective mitral valve endocarditis and its successful repair with one year of follow-up.
Yours sincerely,


