Abstract
Irritable bowel syndrome (IBS) is a common functional bowel disorder. Up to 96% of IBS patients experience bloating, resulting in poor response to conventional therapies and high consultation rates. Many IBS patients report that food triggers symptoms, particularly diets with poorly absorbed, short-chain carbohydrates, and restrict intake of certain foods to control their symptoms. IBS patients are especially susceptible to an attack due to visceral hypersensitivity. An emerging therapeutic strategy excludes fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) from the diet. There is evidence supporting the efficacy of a low FODMAP diet in improving symptoms of bloating in IBS patients. Individualised, structured dietary guidance may benefit those with persistent troublesome symptoms despite traditional therapies. In view of the multifactorial aetiology of the condition, it is probably best to use a multipronged approach, involving combination therapies, to address bloating in IBS patients.
INTRODUCTION
Irritable bowel syndrome (IBS) is a common functional gastrointestinal tract disorder that is characterised by chronic and relapsing bloating, abdominal distension, abdominal discomfort or pain, flatulence, and altered bowel habits with no abnormal pathology. It can be diagnosed using the Rome III criteria. Prevalence rates of IBS vary from 2.9% to 30%(1-3) and its pathogenesis remains largely elusive. A myriad of hypotheses(4) on the pathophysiological mechanism of IBS has been proposed, including altered intestinal mobility, visceral hypersensitivity, altered intestinal permeability, food intolerance, abdominal brain-gut interaction,(5) imbalance of intestinal microbiota, and post-infectious and/or microscopic inflammatory changes.
Bloating is the most bothersome symptom that IBS patients experience. The symptoms of bloating are abdominal fullness and tightness, which is signified by abdominal distention. A majority of patients experience moderate or severe symptoms. Up to 96% of IBS patients(6) experience bloating, compared to 20%–30% of the general population.(7,8) Approximately 50% of patients with bloating also experience an increase in abdominal girth of up to 12 cm. Bloating has a significant impact on quality of life (QOL)(9) and brings about great psychological distress.
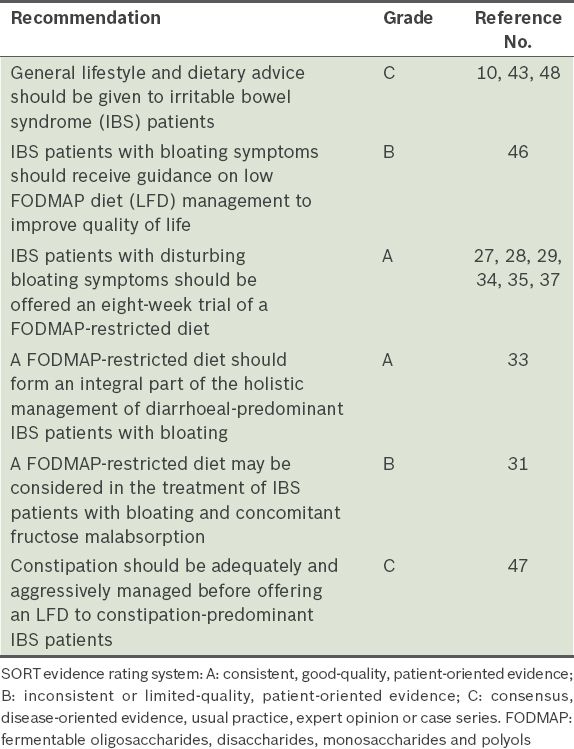
Published guidelines(10,11) recommend the use of antidiarrhoeals, laxatives, fibre supplements and high-fibre diets to improve transit disturbances; and antispasmodics or low-dose antidepressants for abdominal pain. However, these therapies have been unsatisfactory, and largely confer only symptomatic and transient relief. Up to 50% of IBS patients experience postprandial exacerbation of symptoms.(12,13) Approximately 60%–80% of IBS patients believe that their symptoms are diet-related, of which 75% of symptoms are related to incompletely absorbed carbohydrates.(14) Many patients restrict their intake of certain foods to control their symptoms and are interested in the role of diet in IBS.(15-17)
Affluent countries see high consultation rates for IBS, with up to half of patients presenting to and being managed in primary care clinics. This corresponds to an increase in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) intake (especially fructose) over the past three decades,(18) due to the increased availability of concentrated fruit juices and extensive use of high-fructose corn syrup in a wide variety of processed foods and beverages. Earlier studies(19,20) on individual sugars and carbohydrates alluded to sugar malabsorption as a contributor to IBS symptoms; a significant reduction in IBS symptoms has been noted in individuals on a low FODMAP diet (LFD). Dietary, poorly absorbed, short-chain carbohydrates, collectively termed FODMAP, are found in a wide variety of foods, including those containing lactose, fructose in excess of glucose, fructan, galacto-oligosaccharides and polyols (i.e. sorbitol, mannitol, xylitol and maltitol). Ingestion of FODMAP increases delivery of readily fermented substrates and water to the distal small intestine and proximal colon, causing luminal distension and functional gut symptoms. The FODMAP concept hypothesis is that a global restriction should be more efficacious than a limited one in controlling IBS symptoms.
While bloating is a supportive symptom for a diagnosis of IBS, the Rome III diagnostic criteria for functional gastrointestinal disorders do not include bloating as a primary criterion for IBS because of its non-discriminatory nature. Up to 50% of patients who present with bloating do not fulfil the Rome III diagnostic criteria for IBS.(21) As a result, despite the clinical relevance of this symptom, few studies have included bloating as a primary endpoint and, to the best of our knowledge, no studies have specifically examined the efficacy of an LFD in the management of bloating. This review article systematically explores the available evidence to provide recommendations for managing bloating symptoms in IBS patients on an LFD.
METHODOLOGY
Literature search
A primary search of two electronic databases, PubMed and the Cochrane Central Register of Controlled Trials, was conducted using the search terms ‘irritable bowel syndrome’, ‘FODMAP’, ‘FODMAPs’, ‘fermentable oligosaccharides disaccharides monosaccharides and polyols’ or ‘short chain fermentable carbohydrates’ as keywords, and ‘exploded’ medical subject headings, when possible. The search was conducted from September 2014 to June 2015. Limits were applied for non-human studies and articles concerning children. A list of 237 articles was generated. A supplementary search of the archive of referenced articles yielded another three articles.(22,23,24) This reference list of citations was screened based on appropriateness of the study title; 197 articles, including ten non-English articles and two duplicates, were excluded. The full text of the remaining 40 studies was extracted and reviewed for inclusion.
Inclusion criteria
English-language articles on FODMAP, symptoms and bloating in IBS patients were included. As dietary FODMAP composition is likely to vary with the type of cuisine, evidence from meta-analysis, systematic reviews, trials and case studies was evaluated to obtain a better understanding of an LFD in different cuisines. Expert opinions from the consensus guideline and position statement on this matter were also consulted. The primary outcome evaluated was improvement in bloating, while secondary outcomes were improvement in global or composite endpoint symptoms and QOL. Articles were excluded if the LFD was administered through the enteral route. Trials evaluating the effect of LFDs on healthy participants, inflammatory bowel disease, ileostomates and microbiota were not included. Randomised controlled trials (RCTs) were evaluated for risk of bias using the Jadad score (
Table I
Summary of relevant papers.
RESULTS
Papers selected
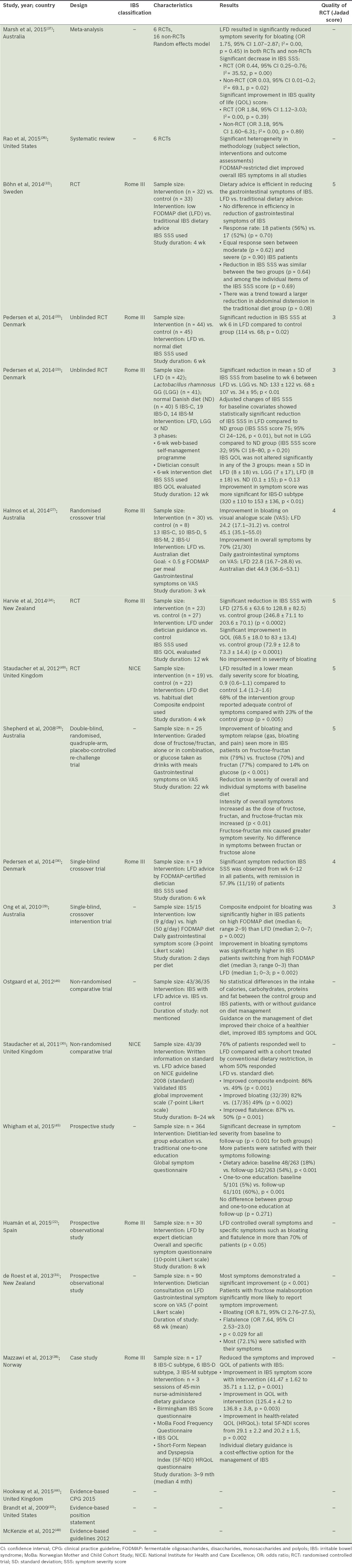
Owing to the nature of the subject matter, there were limited high-quality trials. A substantial proportion of the available literature were narrative reviews. 20 articles were used for this review, comprising one meta-analysis, one systematic review, nine RCTs, two non-randomised comparative studies, three prospective trials, one case study and three guidelines.
Bloating has been included as part of a composite endpoint or as a secondary endpoint in studies targeted at IBS symptoms. Data from these studies were reviewed. Seven out of the 17 studies evaluated bloating as a secondary outcome,(6,22,27-31) while the majority evaluated bloating as a composite endpoint. The IBS symptom severity score (SSS) was used in five(23,24,32-34) of the ten studies that evaluated bloating as part of a composite endpoint, while the other ten studies used a varying visual analogue scale (VAS) or Likert scale.
LFD improves bloating symptoms in IBS
A significant reduction in bloating, either as a composite or secondary endpoint, was observed in most cohort studies and RCTs that used an LFD. Favourable response rates of 70% or more were reported.(22,27,29,30,35) Harvie,(24) interestingly, reported no improvement in the severity of bloating despite a significant reduction in IBS SSS.
A systematic review(36) reported overall improvement in IBS symptoms with a FODMAP-restricted diet. Recently, a meta-analysis(37) of pooled RCTs reported significant reduction in IBS SSS [odds ratio (OR) 0.44, 95% confidence interval (CI) 0.25–0.76; degree of heterogeneity (I2) = 35.52, p = 0.00] and symptom severity for bloating (OR 1.75, 95% CI 1.07–2.87; I2 = 0.00, p = 0.45). Pedersen et al(33) noted a significantly greater improvement in SSS for the IBS-diarrhoea (IBS-D) subtype (320 ± 110 to 153 ± 136, p < 0.01), although IBS-associated bloating is known to be more pronounced in cases with constipation. Patients with fructose malabsorption were also significantly more likely to report symptom improvement (OR 8.71, 95% CI 2.76–27.5).(31)
LFD improves satisfaction with symptom control and QOL
Satisfaction with symptom control increased(22) with better control of bloating symptoms. In one study, 72% of LFD patients were satisfied with their symptom control.(31) Improvement in QOL was also observed with an LFD.(24,33,38) Meta-analysis data(37) reported a significant improvement in the IBS-QOL score (OR 1.84, 95% CI 1.12–3.03; I2 = 0.00, p = 0.39).
Graded dose response
The response to FODMAP restriction corresponded to its dose. The composite endpoint for bloating was significantly higher in IBS patients on a high FODMAP diet (median 6, range 2–9) than an LFD (median 2, range 0–7; p = 0.002).(29) Similarly, improvement in bloating symptoms was significantly higher in IBS patients switching from a high FODMAP diet (median 3, range 0–3) than an LFD (median 1, range 0–3; p = 0.002).(29) Shepherd et al(28) reported that the intensity of overall symptoms increased significantly as the dose of fructose, fructan and fructose-fructan mix was increased. However, it is unclear what the optimal dose and composition of an LFD should be. Halmos et al(27) limited FODMAP intake to no more than 0.5 g per meal. Ong et al(29) set the limit for an LFD at 9 g/day. Most other studies in the literature made no mention of the exact content of the LFD.
Differential effect of FODMAP types
The fructose-fructan mix caused greater symptom severity; but there was no difference in symptoms between fructan and fructose alone. Improvement of bloating and symptom relapse (bloating, gas and pain) was seen more in IBS patients on the fructose-fructan mix (79%) compared to patients on fructan (77%), fructose (70%) and glucose (14%) (p < 0.001).(28)
Time to response and duration of LFD therapy
Time-to-response data varied from 2–8 weeks and symptom reduction was more evident nearer or after six weeks.(33,39) Prompt response to therapy in as early as two days’ time is possible.(28) In individuals for whom osmotic and motility changes are the only mechanisms causing symptoms, rapid response to LFDs (within 24–48 hours) may be expected.
LIMITATIONS
The prospective observational (cohort) studies pointed to a temporal relationship between bloating and FODMAP exposure, while the RCTs provided evidence for causal relationships and supported changes in clinical practice. The RCTs reviewed had a low-to-moderate risk of bias, as assessed by the Jadad score. However, an inherent flaw of the Jadad score is that it does not take into account allocation concealment, which could overestimate the treatment effect by 20%–30%.(40) A reanalysis of the papers revealed that allocation concealment was reported in three(27,29,33) of the nine RCTs. In addition, non-RCT evidence was not critically appraised. Notwithstanding the methodological flaws within the component studies, consistent results were seen across the different studies. In the rechallenge(28) and crossover(27,29,34) trials, patients served as their own controls to evaluate the efficacy of LFDs over a period of time.
Current evidence on the positive effects of FODMAP restriction on IBS-related bloating symptoms and QOL comes from studies with heterogeneous diagnostic criteria, SSS, study design, composition of LFD and duration of follow-up. Different diagnostic criteria abound, with the Rome III criteria being favoured. These studies have little mention of the various IBS subtypes. Different SSSs were employed, with the IBS SSS being the preferred scale in recent years. In addition, the articles included in this paper were limited to those published in English. It would be interesting to examine if the non-English articles suggest the same trends.
DISCUSSION
It appears that IBS patients who may benefit from LFDs include those with (a) bloating who fail to respond to conventional interventions as per treatment guidelines; (b) self-reported bloating symptoms attributed to possible food intolerance, especially fructose intolerance; and (c) IBS-D subtype. As up to half of patients who present with bloating do not fulfil the Rome III diagnostic criteria for IBS,(21) physicians should keep an open mind to exclude other pathologies, such as small intestinal bacterial overgrowth, which could present with bloating.
Evidence points to a favourable response in IBS patients with bloating treated with a FODMAP-restricted diet, and a differential response in terms of FODMAP dose and type. The ideal composition of an LFD is, however, hazy at present. Current researchers have experimented with American, British, Nordic, Australian and New Zealand cuisines. Limited literature regarding non-Western cuisines, such as Asian, is a prevailing issue. Data from non-English (Korean) manuscripts would add invaluable knowledge to this arena. Gwee et al(1) highlighted the following differences in Asian IBS patients: (a) a low female predominance in the majority of Asians; (b) a high prevalence of lactose malabsorption amongst Asians; (c) faster intestinal transit times in both healthy and IBS patients in some Asian populations compared to the West; and (d) chilli consumption, in particular, as an aggravating dietary trigger. Evidence for the association between chilli and symptom aggravation was, however, weak. Considering these differences, Asian patients are likely to respond differently. That said, with globalisation, the Western diet has infiltrated many Asian countries. Diseases, such as inflammatory bowel diseases, that were once thought to be limited to the Western world, are quickly becoming a norm in Asia.(41,42) Research into the Asian patient and diets could help to shed light in this area. In addition, research is also needed to determine the FODMAP content of other cuisines and discover more foods with restricted FODMAP content.
In addition to comparing the LFD with traditional cuisines, LFDs were compared with traditional IBS advice,(43,44) which includes (a) having small, frequent meals; (b) to peel and divide foods into pieces; (c) chew thoroughly; (d) boil food; and (e) reduce fatty and spicy foods, legumes, onions, coffee and alcohol. In addition, carbonated beverages and sweeteners containing polyols should be avoided, and fibre intake should be evenly distributed over the day. Böhn et al(32) compared the effects of adopting traditional IBS dietary advice with an LFD, and reported no difference in efficiency regarding the reduction of gastrointestinal symptoms of IBS. Reduction in the IBS SSS was similar between the two groups (p = 0.64) and among the individual items of the IBS SSS (p = 0.69). A trend of a larger reduction in abdominal distension in the traditional diet group was also observed. However, a non-randomised trial reported conflicting results; Staudacher et al(30) reported improvement in bloating [(32/39) 82% vs. (17/35) 49%, (p = 0.002)] when written information on LFDs was compared with standard dietary advice. While further research is needed to draw conclusions, the FODMAP-restricted diet still has a role in alleviating bloating symptoms in IBS patients.
The National Institute for Health and Care Excellence guidelines(44) strongly advocate self-help, structured patient education and other non-pharmacologic strategies such as lifestyle and diet modification. Previous studies have established the importance of offering education, information and general support in the management of IBS. Given individually or to a group,(38,45) advice and guidance on FODMAP restriction is a cost-effective therapy in the management of IBS-related bloating. Nevertheless, the ideal mode and medium for patient education remain debatable. While most trials involved a FODMAP-certified dietitian, a nurse-led dietary guidance consisting three 45-minute sessions(38) resulted in a favourable symptom response (41.47 ± 1.62 to 35.71 ± 1.12; p = 0.001); hence, written information and a food list on LFD choices may be as effective as a dietitian consult. Improvement in bloating was reported by Staudacher et al,(30) who evaluated written information on LFDs versus ‘standard’ diets (82% vs. 49%; p = 0.002). In de Roest et al’s(31) study, 18.2% of the subjects felt that a food list was as effective as a dietitian consult. Web-based self-management programmes have also proven to be feasible approaches to chronic diseases. A significant reduction in symptom severity was reported by Pedersen et al(33) when a Web-based application was utilised to provide education, disease recognition and general support prior to dietitian-guided LFDs.
Hitherto, no validated biomarkers have been established for objective measurement of predominant IBS symptoms such as bloating. Consequently, patient-reported outcomes of symptom severity were relied on to evaluate symptom improvement. The validated IBS SSS(35) appeared to provide a reliable measure of the overall severity of IBS. It contains five questions that measure bloating, among other symptoms, on a 100-point VAS. Presented in a traffic light system (Appendix) similar to the Written Asthma Action Plan, the IBS SSS serves as a simple tool to assess symptom severity and monitor response to therapy. In the traffic light system, red indicates severe symptoms, yellow moderate symptoms and green mild symptoms or remission.
Across trials, the adherence rate had a range of 20%–40%, illustrating patients’ difficulty with this restrictive diet. This is not surprising, given the highly intensive demands of an LFD. Factors such as ease of finding suitable food products, palatability of the diet, cost, patient understanding and personal motivation contribute to the plausibility and success of such a management strategy.(22,28)
The relatively short duration (range 154–251 days) of recently published studies did not allow sufficient time to gauge the sustainability of the effect of dietary changes. Concerns have been voiced about the nutritional value and adequacy of the LFD, although minimal adverse reactions have been reported. It is reassuring to note that no statistical difference in the intake of calories, carbohydrates, proteins and fat has been reported between the control and IBS patients.(35,46) However, FODMAPs have prebiotic effects due to the production of short-chain fatty acids after fermentation. Long-term effects of LFDs on the gut microbiota and nutritional state of patients have yet to be evaluated.
Slightly more than one-third of the patients in Pedersen et al’s study received conventional IBS medication, such as laxatives, antispasmodic and antidiarrheal agents, and antidepressants. Patients on IBS medication and LFDs responded significantly better than patients on LFDs alone. The improved response to combined therapy could be due to the complexity of IBS symptoms and the natural disease course of IBS, which requires medication that is effective against specific symptoms.(33) Constipation-predominant IBS requires initial treatment aimed at ensuring that constipation is adequately and aggressively managed.(47)
The findings in this paper may represent the biased view from a single reviewer. Retrospectively, we could have involved a second independent reviewer to perform the literature search and a third-party arbitrator to address any disagreement, to ensure the robustness of this paper. Nonetheless, there is a consistent trend suggesting that LFDs improve bloating symptoms in IBS patients.
CONCLUSION
The LFD approach to the management of IBS patients is an emerging therapy. Current evidence supports the efficacy of LFDs in improving IBS-related bloating symptoms, which is a patient-important outcome. IBS is a chronic condition and, as in most chronic illnesses, awareness and education can empower the patient, foster self-efficacy in symptom control and liberate the IBS patient. Individualised, structured dietary guidance may benefit those with persistent, troublesome symptoms despite traditional therapies. However, given the multi-factorial aetiology of bloating in IBS, a multipronged approach involving a plurality of therapies is probably prudent.
Supplementary Material
SMJ-57-484-Appendix.pdf