Abstract
INTRODUCTION
We aimed to review the necessity of conventional interventions in renal transplant for preventing complications arising out of the use of wound drains, ureteral stents and stapled skin closures.
METHODS
We reviewed a series of 33 patients who received stentless, tubeless/drainless and suture-apposed living donor renal transplants (STAR group) and compared the results to a control non-STAR group of 36 patients in whom all three interventions of drains, stents and skin staples were used.
RESULTS
No significant differences in demographics and clinical characteristics were observed between the two groups. With regard to the overall surgical complications, no significant differences in terms of wound infection, seroma, perinephric collections, urinoma, bacteriuria or vascular complications were observed between the groups. When analysed according to the interventions specific for preventing complications, although slightly more asymptomatic perinephric collections were observed and two lymphoceles required treatment in the STAR group, these differences were not statistically significant. Similarly, no significant differences in ureteric or skin-related complications were observed between the groups. Both groups had comparable good outcomes for renal function, graft survival and patient survival.
CONCLUSION
The routine use of ureteric stents, drains or skin staples may not be necessary for uncomplicated renal transplants. Potential complications associated with the placement of these interventions can be avoided without compromising on the safety of patients and/or the outcome of transplants.
INTRODUCTION
Renal transplantation is the optimal renal replacement therapy for patients with end-stage renal failure. There has been a move towards reducing the morbidity of conventional open transplant surgery with the use of minimally invasive renal transplantation with or without robotic assistance.(1) However, this procedure is technically challenging and expensive, resulting in its limited uptake by mainstream transplant centres. There may be other technical modifications that are in line with the worldwide trend towards reducing surgical morbidity. Conventional surgical techniques include the use of retroperitoneal wound drains, ureteral stents and stapled skin closures to prevent lymphoceles, ureteric complications and wound complications, respectively.(2) These may not be necessary in uncomplicated renal transplants. In keeping with this trend, the National University Hospital (NUH), Singapore, started a wound management programme known as the STAR (stentless, tubeless, apposed renal) transplant programme in 2015. This study aimed to report the outcomes following the initiation of this systematic programme to reduce transplant-related surgical morbidity by reducing the use of stents, tube drains and stapled skin closures for a select group of patients.
METHODS
All patients who underwent living donor kidney transplantation at NUH were included in this analysis. Data was collected retrospectively from our institutional transplant database from two consecutive series of patients who underwent living donor renal transplantation between 2013 and 2016, which was approved by the institutional review board. Patients were excluded if they were aged less than 18 years and were recipients of multiple organ transplants or given sirolimus immunosuppression. The use of sirolimus had been stopped at our institution since 2010 owing to its association with a higher incidence of complications, including de novo wound complications and, specifically, postoperative formation of lymphoceles.(3,4)
Transplant recipient surgery was performed through an extraperitoneal Gibson incision, with creation of standard vascular anastomoses and extravesical ureteroneocystostomy. All recipients were administered prophylactic broad-spectrum parenteral antibiotics at the time of transplant. Patients were placed in either of the two study groups, STAR and non-STAR. The non-STAR control group consisted of patients who underwent living donor kidney transplantation throughout the study period and had conventional surgical site interventions. These consisted of a stented (Size 6Fr, 16 cm; Cook Medical LLC, Bloomington, IL, USA) extravesical ureteroneocystostomy and a retroperitoneal closed suction drain (size 10 Ethicon Blake drain; Ethicon US LLC, Bridgewater, NJ, USA) placed at the time of transplant. Wound closure involved a multilayer closure, with the skin closed using skin staples (
Fig. 1
Photograph shows a patient from the non-STAR group with complicated or repeat transplant and chronic immunosuppression. STAR: stentless, tubeless, apposed renal transplant
STAR transplant recipients comprised patients who underwent living donor renal transplantation from 2015 till the end of the study period. For patients who underwent transplantation during this period, those with a body mass index (BMI) < 32 kg/m2, those undergoing first transplants, those not on chronic steroids, those without previous pelvic surgery or radiation, and those without intraoperative complications, transplant surgery was performed without the placement of ureteric stents or insertion of retroperitoneal closed suction drain, and/or with skin closure using subcuticular Monocryl 3/0 sutures (Ethicon US LLC, Cincinnati, OH, USA) (
Fig. 2
Photograph shows a patient from the STAR group with uncomplicated first transplant. STAR: stentless, tubeless, apposed renal transplant
The decision regarding the non-use of all three interventions or any stent, wound drain or skin staples for the wound management of patients in the STAR group depended on the preference of the operating surgeon. All recipients from both groups had undergone regular pelvic ultrasonography during postoperative outpatient visits and in case of episodes of renal dysfunction, at ureteral stent removal and for wound-related complications. Pelvic computed tomography imaging was performed more selectively.
All transplant recipients in our study were given a non-depleting interleukin-2 receptor-blocking antibody, basiliximab (Simulect; Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA), or a polyclonal rabbit anti-T-cell induction antibody (Thymoglobulin; Sanofi SA, Paris, France) as induction. For maintenance of immunosuppression, all patients received cyclosporine or tacrolimus, mycophenolate mofetil (CellCept; Hoffmann-LaRoche, Nutley, NJ, USA) and steroids, according to institutional protocols. In both patient groups, cyclosporine and tacrolimus were introduced to maintain 24-hour trough C0 levels of 250–375 mg/L and 1.8–4.0 mg/mL, respectively, during the perioperative period. Mycophenolate mofetil was started at a fixed dose of 1 g twice daily. Dose adjustments for side effects, such as bone marrow suppression or gastrointestinal toxicity, were made, as required. Intravenous hydrocortisone 1 g was administered on Days 0 and 1 to all patients. Doses of steroids were tapered systematically to a maintenance dose of 0.1 mg/kg over three months for most patients.
Each transplant patient was analysed for the incidence, symptoms and treatment of post-transplant vascular complications, wound complications and fluid collection. All available medical records, including imaging studies, were reviewed for the diagnosis of prolonged wound healing and post-transplant fluid collection greater than > 3 cm. The repair of wounds and lymphoceles, including all open and minimally invasive surgical methods, and the medical and nursing treatment of wound complications were recorded. A wound was considered healed with no complications if all the suture material and staples were removed and the wound was intact without drainage by three weeks after transplant. Any wound that opened, drained fluid, dehisced, herniated or became infected beyond this point was considered not healed and registered as a wound complication.(3)
Various demographic and clinical characteristics that are known to affect transplant outcomes were compared between the two groups. These included the recipient age, BMI at the time of transplantation, gender, ethnicity, aetiology of renal failure, presence of diabetes mellitus, type of immunosuppression used, presence of delayed graft function (as indicated by the need for haemodialysis within the first postoperative week) and acute rejection episodes. Outcome measures recorded included the rates of overall wound and specific surgical site complications, need for secondary surgical repair or nonoperative management of wounds, frequency of lymphocele detection, and the number and types of interventions required to treat lymphoceles. The indications for lymphocele treatment included persistence of fluid collection despite a prolonged period of conservative management or drainage, renal dysfunction, urinary frequency from bladder compression, wound swelling and pain, ipsilateral leg oedema and fluid leakage through the wound.
Categorical variables (gender, ethnicity, presence of diabetes mellitus, aetiology of renal failure, use of depleting and maintenance immunosuppressants, and the need for postoperative haemodialysis) were analysed using chi-square test or Fisher’s exact test for small samples. Continuous variables (age, BMI, and postoperative creatinine and estimated glomerular filtration rate levels), if normally distributed, were analysed using an unpaired t-test. If the distributions were not normal, Wilcoxon signed rank test was used. All significance tests were two-sided, at a = 0.05. STATA version 14.0 (StataCorp, College Station, TX, USA) was used to perform all statistical analyses.
RESULTS
The demographics of the patients in the two groups (STAR and non-STAR) are presented in
Table I
Comparison of demographics and clinical features between patients in the two groups.
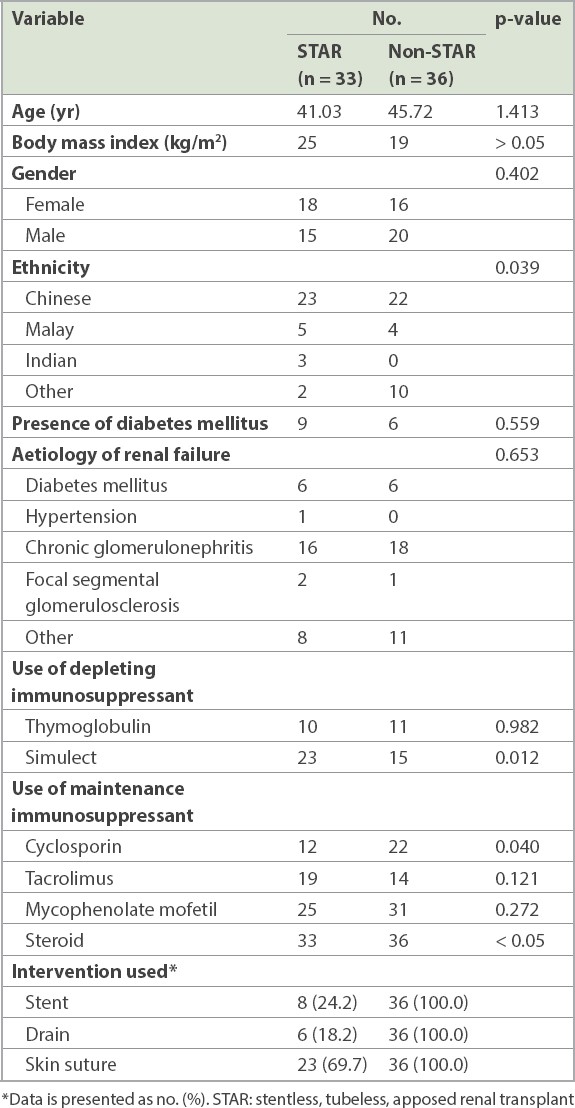
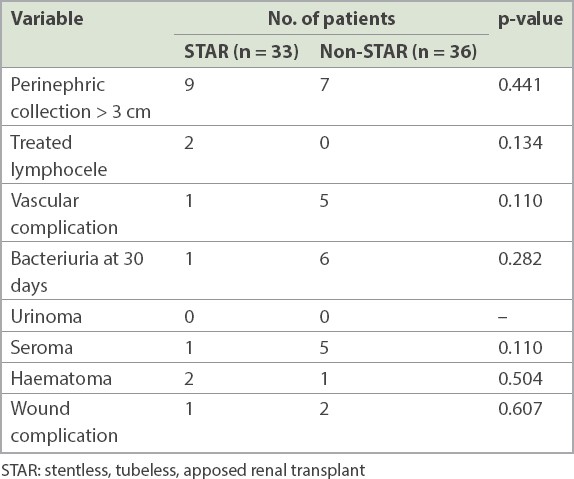
Table II
Comparison of overall surgical site complications.
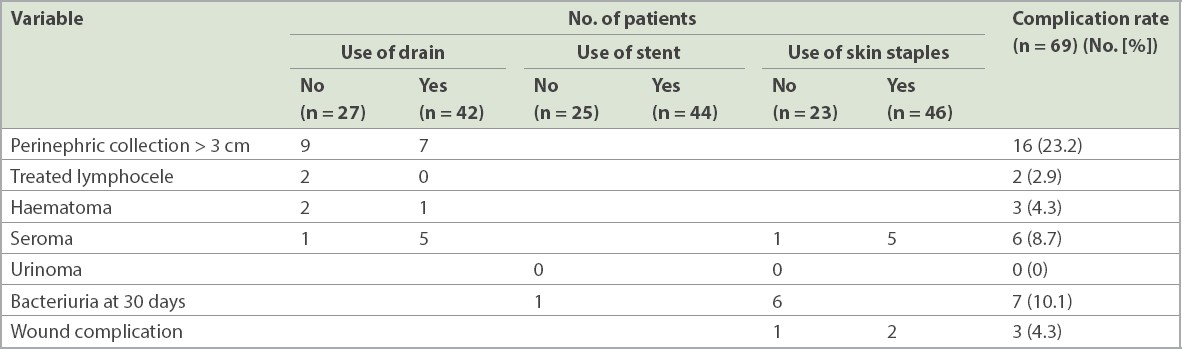
As patients in the STAR group were selectively managed with the non-use of one or all three interventions of stents, drains or skin staples, we reviewed the rates of surgical site complications with regard to the different conventional surgical site interventions specific for preventing complications (
Table III
Comparison of surgical site complications by intervention used.
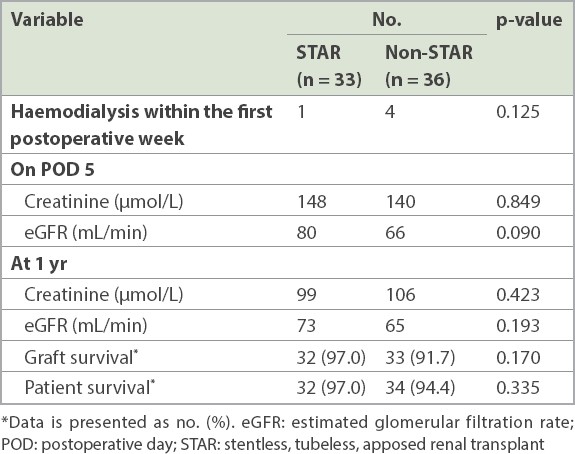
A comparison of the functional outcomes between the STAR and non-STAR groups (
Table IV
Comparison of functional outcomes between the groups.
DISCUSSION
With an aim to reduce the surgical morbidity associated with kidney transplantation, the present study showed that modification of the surgical technique by simplifying surgical site interventions to reduce the use of stents, drains and surgical staples did not result in a significant increase in surgical site complications among selected patients. Specifically, not routinely using ureteric stents, retroperitoneal drains or skin staples did not result in a corresponding increase in ureteric complications, deep (lymphocele) or superficial (seroma) wound collections, or infections, respectively.
This is consistent with recent research efforts directed at challenging or modifying the conventional open kidney transplantation technique, such as recent publications on minimally invasive kidney transplantation.(1) While robotic or laparoscopic kidney transplantation can only be performed at high-volume centres at high cost, with no evidence of improved outcomes as yet, our study described relatively simple and less radical changes that can be performed at most centres, with reduction in morbidity and costs.(4) Although the use of ureteric stents, drains and skin closure with staples have been shown to reduce surgical site complications among transplant recipients with high risk factors, including high BMI (> 32 kg/m2), de novo sirolimus immunosuppression and defunctioned bladders, we questioned the ‘one size fits all’ approach for all patients undergoing transplantation, especially the low-risk, living donor transplant recipients.
For uncomplicated renal transplants, the use of routine stenting, drain placement and/or skin staples may not be associated with any significant morbidity or complications, with good functional outcomes. Although the placement of ureteric stents across the vesicoureteric anastomosis during transplantation has been shown to significantly reduce complications, several centres have chosen to selectively perform stenting when complications may be expected. Dharnidharka et al(5) reported, in a series of 129 transplants, that patients with stents had rates of urine leak and stenosis similar to those of patients without stents. Sinangil et al(6) suggested that other factors may be important for determining urine leak, including the choice of operative technique, ureteric stripping or injury, multiple renal arteries, damage to lower polar artery, cold ischaemia time and donor vascular disease. Stents are often associated with complications including haematuria, migration, infections, encrustation, fragmentation and ‘forgotten’ stents. Stents can also cause discomfort to the patient by causing lower urinary tract symptoms and bladder pain.(7) In addition, removal of stents requires an additional procedure to be performed, incurring additional costs and resources. Stent removal is commonly performed with flexible cystoscopy under local anaesthesia, potentially exposing the patient to subsequent risks of infection from instrumentation. A recent randomised controlled trial confirmed that early stent removal on Day 5 significantly reduced stent-related complications and improved the quality of life in the first three months after transplantation.(8) A Cochrane review by Wilson et al,(7) including 1,154 patients, found that patients who had ureteric stents developed more complications of haematuria, irritative symptoms and stent-related pain. Two grafts were lost as a result of stent-related infectious complications, and pooled results also indicated a general increased risk of urinary tract infection due to the use of stents. By extrapolating the outcome of this earlier trial, it was expected that the absence of stents in our patients would eliminate stent-related complications in the absence of ureteric complications. Our study also supported the evidence that compared with routine stenting of the uretero-neovesical anastomosis, elective stenting does not result in an increase in ureteric stricture or leak for low-risk, living donor kidney transplants.
Wounds closed with sutures have been shown to have a lower rate of wound dehiscence and composite wound complications compared with wounds closed with skin staples, as reported in the study by Clay et al.(9) In a multicentre randomised controlled trial by Imamura et al(10) on the use of subcuticular sutures versus staples for skin closure after open abdominal surgery, the authors concluded that subcuticular sutures did not increase the occurrence of superficial surgical site infections following open laparotomies mainly consisting of clean-contaminated surgical procedures. In our study, a lower incidence of wound complications was detected in the STAR group than in the non-STAR group, although the difference did not reach statistical significance. In previous studies, including the study by Tiong et al,(3) delayed wound healing was observed in patients with a higher BMI or in those who had received sirolimus-based immunosuppression, and among those with other known risk factors for wound healing, including diabetes mellitus, chronic steroid use and prior pelvic surgery or radiation. We also postulated that this finding of delayed wound healing would be applicable to patients receiving everolimus-based immunosuppression or any immunosuppressants belonging to the mammalian target of rapamycin inhibitor group, as they belong to the same class of drugs as sirolimus. By individualising our wound management approach to exclude patients with the above high-risk factors from STAR transplantation, surgical site complications, including infections, did not significantly differ between the groups regardless of whether the skin was closed with sutures or staples.
The placement of a closed suction retroperitoneal drain was also reported to reduce the risk of formation of clinically significant lymphoceles in the transplant bed for high-risk patients. Tiong et al(3) previously reported a 38.7% incidence of lymphoceles in 307 patients, 17.9% of whom required treatment; nearly half of these patients required operative treatment. In our present study, a slightly higher number of patients with lymphoceles > 3 cm was detected among patients without drain placement, and two patients required treatment for lymphoceles owing to prolonged persistence of the lymphocele. However, these differences were not statistically significant, and the lymphoceles did not compromise or affect any graft function. Systematic reviews and meta-analyses of studies on patients undergoing other abdominal surgical procedures have also not shown any benefit of the routine use of abdominal drains after transplant, without any significant difference in the rates of superficial surgical infections.(3,11) By contrast, drain placement was associated with complications such as pain, foreign body reactions and the formation of enterocutaneous fistulae.(11,12)
The non-use of stents, drains or surgical skin staples has been individually reported to be safe for open kidney transplantation.(5-8) Our study uniquely combined the selective use or non-use of these interventions into a wound management programme for kidney transplantation that was individualised for patients depending on their risk factors.
Our study has some limitations. Although this was a prospective study, we acknowledge that the non-use of stents, drains and skin staples was applied selectively by the surgeon and thus, there was inevitable selection bias. However, this likely reflects real-life practice and, importantly, shows that wound management in kidney transplantation can be individualised according to the risk factors of the patients rather than continuing to adhere to a ‘one size fits all’ treatment approach. In addition, while the number of patients in our preliminary study was small, in our opinion, the reported outcomes of our study were adequate to determine that patient safety would be ensured if this inexpensive wound management programme to reduce surgical morbidity associated with open kidney transplantation is adopted by other centres in the long term.
About the First Author

Dr Melissa Tay is a consultant in the Department of Urology at the National University Health System, Singapore. She graduated from the Yong Loo Lin School of Medicine, National University of Singapore, Singapore, in 2010 and obtained post-graduate qualifications from the Fellow of the Royal College of Surgeons and Physicians (Glasgow). Dr Tay is also a faculty at the Department of Surgery, Yong Loo Lin School of Medicine, and participates in various undergraduate and postgraduate education programmes. She manages general urology conditions, with a special interest in adolescent transitional care urology, female, functional and reconstructive urology.


