Abstract
INTRODUCTION
In this study, we report our initial experience with robotic hepatectomy.
METHODS
Consecutive patients who underwent robotic hepatectomy at Singapore General Hospital, Singapore, from February 2013 to February 2015 were enrolled in this study. The difficulty level of operations was graded using a novel scoring system for laparoscopic hepatectomies.
RESULTS
During the two-year period, five consecutive robotic hepatectomies were performed (one left lateral sectionectomy, one non-anatomical segment II/III resection, one anatomical segment V resection with cholecystectomy, one extended right posterior sectionectomy and one non-anatomical segment V/VI resection). Two hepatectomies were performed for suspected hepatocellular carcinoma, two for solitary liver metastases and one for a large symptomatic haemangioma. The median age of the patients was 53 (range 38–66) years and the median tumour size was 2.5 (range 2.1–7.3) cm. The median total operation time was 340 (range 155–825) minutes and the median volume of blood loss was 300 (range 50–1,200) mL. There were no open conversions and no mortalities or major morbidities (> Clavien-Dindo Grade II). The difficulty level of the operations was graded as low in one case (Score 2), intermediate in three cases (Score 5, 6 and 6) and high in one case (Score 10). There was one minor morbidity, where the patient experienced Grade A bile leakage, which resolved spontaneously. The median length of postoperative hospital stay was 5 (range 4–7) days.
CONCLUSION
Our initial experience confirmed the feasibility and safety of robotic hepatectomy.
INTRODUCTION
Since its introduction in the late eighties, laparoscopic surgery has been increasingly and rapidly adopted in the field of abdominal surgery. Today, in many tertiary centres, laparoscopic surgery is performed on an almost routine basis for abdominal surgical procedures such as appendectomy,(1) cholecystectomy,(2,3) adrenalectomy,(4,5) gastric resection(6) and colectomy.(7) However, the adoption of laparoscopy in hepatectomies has been limited until the last decade, despite the widespread use of laparoscopy in many abdominal procedures;(8) this is due to the technical complexity of the procedure, and concerns regarding the adequacy of oncological margins and the risk of bleeding.(9,10)
The rapid development of more sophisticated laparoscopic instruments during the past decade has improved the overall safety and reliability of laparoscopic surgery and led to its expansion to more complex operations such as hepatectomies.(11,12) An increasing number of studies have demonstrated that laparoscopic hepatectomy is comparable to conventional open surgery in terms of safety and pathological outcomes, but with the added advantages of laparoscopic surgery.(13-16) However, despite the promising advances in laparoscopic equipment, the inherent limitations of conventional laparoscopy (e.g. restriction in movement and dexterity due to the rigidity of instruments, tremor amplification and lack of depth perception) remain a major hindrance to the adoption and widespread application of laparoscopy in complex abdominal operations.(17) One of the major criticisms of laparoscopic hepatectomy is that it has a relatively long learning curve and limited universal applicability.(18)
Robotic surgery was introduced in the 1990s to overcome the limitations of conventional laparoscopic surgery. The robotic platform provides a three-dimensional, high-definition, magnified view of the operative field, as well as improved dexterity and precision (via increased freedom of movement and elimination of tremor).(19,20) However, the superiority of robotic surgery over conventional laparoscopy in performing complex surgical tasks has only been proven in ex vivo models.(19) Presently, the use of robotic technique for hepatectomy remains relatively new and indications for its application remain controversial.(21)
In the present study, we report our experience with the first five consecutive robotic hepatectomies in a single institution. We aimed to determine the feasibility and safety of the procedure. To the best of our knowledge, this is the first reported series of robotic hepatectomies in Singapore.
METHODS
This prospective study was approved by the Review Board at Singapore General Hospital (SGH), Singapore. Five consecutive patients who underwent robotic hepatectomies using the da Vinci® Si surgical system (Intuitive Surgical Inc, Sunnyvale, CA, USA) from February 2013 to February 2015 at SGH were enrolled in this study. Prior to this study, the surgeons at our institution had the collective experience of more than 2,500 hepatectomies, including over 150 laparoscopic hepatectomies, since the year 2000.
The anatomical location of the lesions and surgical resection were defined according to the Couinaud classification.(22) Liver resections were graded according to the level of difficulty of the laparoscopic resections, based on a novel scoring system proposed by Ban et al, using an index of 1–10: low difficulty 1–3; intermediate difficulty 4–6; and high difficulty 7–10.(23) In this novel scoring system, the difficulty level of a laparoscopic hepatectomy was scored based on several factors, including the type of resection, location of lesion, size of tumour and proximity of the tumour to major vessels. The choice of surgical approach – laparoscopic, robotic or open – depended on various factors, including the individual surgeon's preference and the patient's choice after thorough discussion of the benefits and limitations of the different approaches. In the present case series, two principal surgeons conducted the five surgeries; Goh BK performed on four cases, while Lee SY performed on one case.
The patients were placed in the reverse Trendelenburg position for surgery. Port placements were similar for all patients, with slight variations made according to the position of the patient's tumour. In general, a 12-mm trocar was inserted in the midline above the umbilicus via the Hassan technique for the robotic camera. Three 8-mm robotic working ports were inserted under laparoscopic guidance at the right upper quadrant, upper midline and left upper quadrant. In the first three cases (Cases 1–3), only two robotic working ports were used and a 12-mm trocar was placed at the right abdomen lateral to the umbilicus for the bedside assistant. In selected cases, an additional 5-mm assistant port and/or a 5-mm epigastric port was inserted for the Pringle manoeuvre, if deemed necessary. The robotic system was brought into position over the patient's left or right shoulder, depending on the tumour location, and docked after placement of the ports. The assistant surgeon was positioned between the patient's legs.
Laparoscopic intraoperative ultrasonography was routinely performed and liver parenchymal transection was performed using robotic harmonic scalpel, monopolar cautery hook/scissors and fenestrated bipolar diathermy forceps. Liver parenchymal transection was performed with the harmonic scalpel using a combination of clamp-crushing or direct sealing/cutting techniques. Conventional laparoscopic instruments were used by the assistant for retraction, suction or application of clips. In the remaining two cases (Cases 4 and 5), a laparoscopic cavitron ultrasonic surgical aspirator (CUSA; Valleylab, Boulder, CO, USA) was used to assist in parenchymal transection. After the resection was completed, the specimen was extracted through an extension of the supraumbilical or assistant port site using the Endobag™. Operative (including docking) time, total volume of operative blood loss and length of postoperative hospital stay after surgery were recorded. Any postoperative complications encountered up to six months after the surgery were recorded using the Clavien-Dindo classification.(24)
RESULTS
During the study period, five consecutive patients underwent robotic hepatectomy. The type of hepatectomy performed and the indications for hepatectomy are as follows: anatomical left lateral sectionectomy for a haemangioma (n = 1); non-anatomical segment II/III resection for a hepatocellular carcinoma (n = 1); anatomical segment V resection with cholecystectomy for a focal nodular hyperplasia (n = 1); extended right posterior sectionectomy for breast liver metastasis (n = 1); and resection of segments V/VI for colorectal liver metastasis (n = 1).
The characteristics of the five patients are summarised in
Table I
Summary of demographics and clinical outcomes of the five patients who underwent robotic hepatectomies.
Case 1
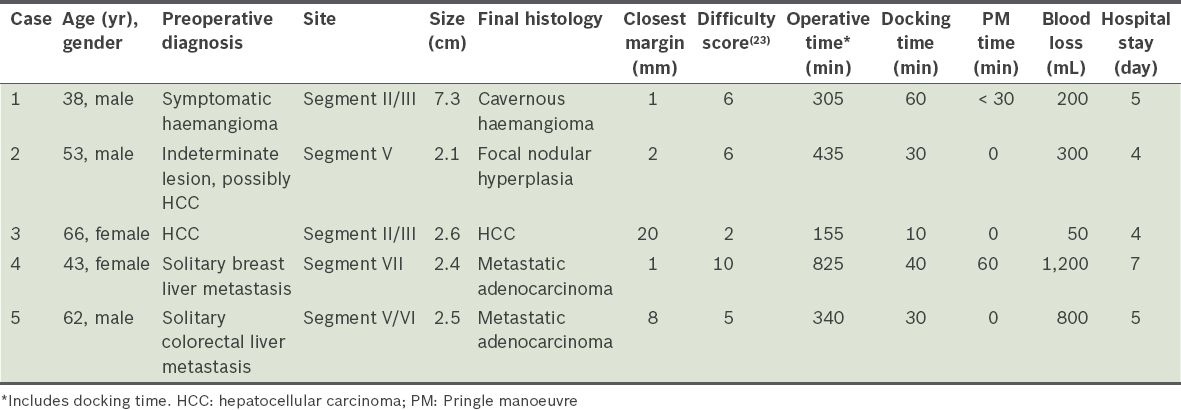
The first case, performed in February 2013, involved a 38-year-old man who presented with abdominal discomfort. He had no significant past medical history. Computed tomography of the abdomen revealed a large segment II/III haemangioma (measuring 7.3 cm at its largest diameter) abutting the left hepatic and portal veins (
Fig. 1
Case 1: CT image of the abdomen shows a large symptomatic segment II/III haemangioma (arrow) abutting the left hepatic vein and portal vein.
Case 2
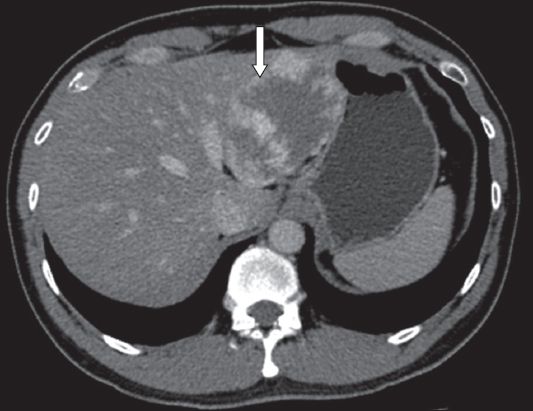
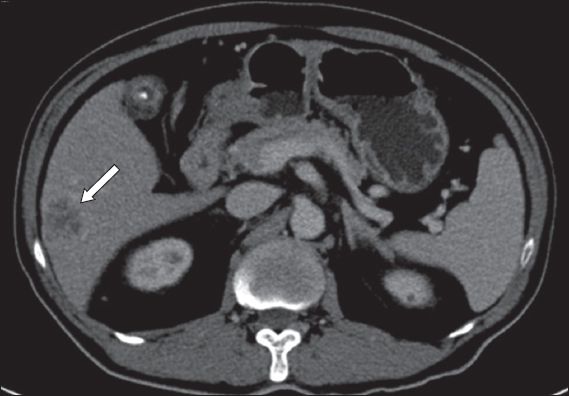
A 53-year-old man with chronic hepatitis B was detected to have a segment V tumour on surveillance ultrasonography. Magnetic resonance (MR) imaging revealed an indeterminate arterial enhancing lesion (measuring 2.1 cm) with atypical washout pattern in the portal venous phase (
Fig. 2
Case 2: MR image of the liver of the patient (who has chronic hepatitis B) shows an indeterminate segment V lesion (arrow).
Case 3
A 66-year-old woman with chronic hepatitis B was found to have a 2.6-cm segment II/III tumour, which was characterised as a hepatocellular carcinoma on MR imaging. She underwent non-anatomical resection of segment II/III. The tumour was confirmed on final histology to be a hepatocellular carcinoma with clear resection margins. The patient recovered well and was discharged on postoperative Day 4.
Case 4
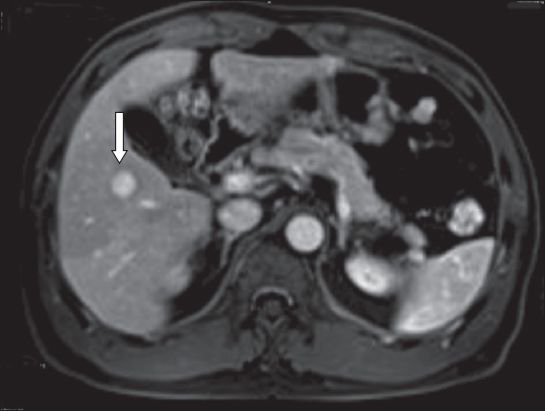
A 43-year-old woman was detected with solitary metachronous liver metastasis after previous mastectomy and adjuvant chemotherapy for breast cancer. MR imaging demonstrated a 2.4-cm segment VII lesion involving the right hepatic vein. The lesion was located close to the junction of the right hepatic vein and inferior vena cava (
Fig. 3
Case 4: MR image of the liver shows a solitary liver metastasis (arrow) at segment VII, close to the root of the right hepatic vein and inferior vena cava.
Case 5
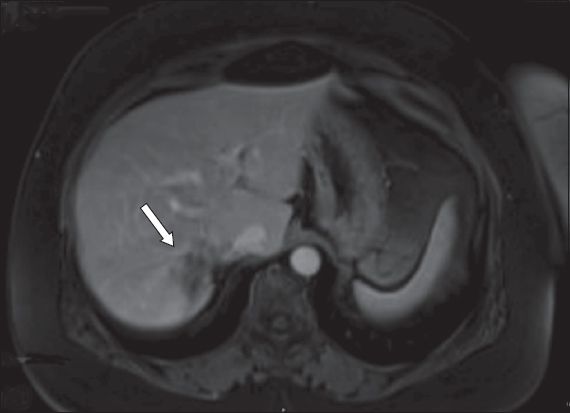
A 62-year-old man presented with synchronous liver metastasis from an obstructing distal colonic adenocarcinoma with gallbladder stone. The patient underwent colonic stenting followed by laparoscopic anterior resection. Despite adjuvant chemotherapy, solitary segment V/VI liver metastasis was noted to have increased in size, from 1.7 cm to 2.5 cm (
Fig. 4
Case 5: MR image of the liver shows a solitary liver metastasis (arrow) at segment V/VI, with gallbladder stone.
DISCUSSION
To date, hepatectomy remains one of the most technically challenging abdominal operations to perform, as the liver is a highly vascularised organ with a unique and complex vasculature and biliary drainage system. Although the mortality rate after liver resections has decreased significantly with improved surgical techniques and perioperative care, morbidity rates remain high.(25) Perioperative haemorrhage and bile leakage are the most common complications after hepatectomy.(26) Due to its technical complexity, it is not surprising that the surgical community has been comparatively slow to adopt the laparoscopic approach for hepatectomies compared to other types of abdominal operations.
In the last decade, the adoption of laparoscopic hepatectomy has increased exponentially worldwide as a result of advancements in surgical technique and laparoscopic equipment.(11,27) Today, its adoption is hindered mainly by the reportedly steep learning curve, especially for major hepatectomies; it is also doubtful whether the promising results reported by expert, high-volume centres are applicable to lower-volume centres. In an early study conducted by pioneering surgeons, it was reported that about 60 cases may be required for a surgeon to achieve competency in laparoscopic hepatectomy.(18) However, more recent studies have reported that the learning curve may be shortened to about 20 cases, especially for minor laparoscopic hepatectomies.(9,28)
Robotic surgery was introduced to overcome the limitations of conventional laparoscopic surgery.(29,30) It has been hypothesised that for experienced surgeons who are only familiar with the open approach, the learning curve for robotic surgery may be less steep than that for conventional laparoscopy.(29-31) This is due to the fact that the robot is built to mimic a surgeon's hands; in other words, open surgical techniques are more readily translated to robotic surgery than laparoscopic surgery.(30)
Despite its many theoretical advantages, robotic surgery is associated with several limitations. Currently, its main limitation is cost, which has been reported to be several times higher than that of conventional laparoscopic surgery.(32) Its high cost has resulted in many surgeons having no or limited access to the technology. In addition, the size and bulkiness of the current robotic platform could result in arm collision during manipulation, limiting the robot's movement. There is also the absence of tactile feedback from the robotic arms, forcing surgeons to rely on experience and imagination during organ retraction and suturing.(33) Furthermore, any change in the position of the patient would require the robot to be re-docked. The surgical and operating room teams need to undergo specialised training in order to dock the robot and exchange the surgical instruments on the robotic arms. Some authors have attributed the time spent docking and changing instruments to be one of the main reasons robotic surgery generally takes longer than conventional laparoscopic surgery, especially during the learning phase.(34) It has been suggested that experience with at least ten robotic procedures is required to master robot-assisted laparoscopic surgery, and that a skilled assistant is needed to handle suction and stapling devices, especially for complex operations.(33)
Presently, robotic hepatectomy is a relatively new procedure with limited studies published worldwide.(33,34) Most of the published studies involved a small retrospective series of patients, and demonstrated that robotic hepatectomy is safe and feasible when performed by experienced surgeons.(31,34,35) Nonetheless, there is evidence to suggest that robotic hepatectomy may be superior to conventional laparoscopy. For instance, a study by Tsung et al demonstrated that patients who underwent robotic hepatectomy were less likely to require conversion to hand-assisted surgery as compared to those who underwent laparoscopic hepatectomy.(34) Another study reported that robot-assisted surgery may be more advantageous than conventional laparoscopic surgery when dealing with intraoperative complications (e.g. major vascular injury) during hepatectomies.(36) Pelletier et al reported in their systematic review that robotic hepatectomy enabled intracorporeal suturing and tying in difficult locations, as well as efficient control and management of bleeding, such that the need for conversion to open surgery was reduced.(35) As with laparoscopic hepatectomy, most reported cases of robotic hepatectomy were performed in highly specialised tertiary referral centres. While most of the aforementioned studies reported promising short-term outcomes, the long-term oncological outcomes and cost-effectiveness of the procedure remain unanswered. Pelletier et al reported that robotic hepatectomy was associated with increased cost and longer operating time as compared to conventional laparoscopic surgery,(35) while a more recent systematic review reported that not only was laparoscopic hepatectomy associated with shorter operating time, but it also decreased the volume of blood loss as compared to robotic hepatectomy.(37) In other words, based on the current literature, there is limited evidence to support the superiority of robotic hepatectomy over conventional laparoscopic hepatectomy.
The results of our initial experience with robotic hepatectomy were consistent with the findings in the literature. In all five cases, robotic hepatectomy was successfully completed without the need for conversion to open or hand-assisted surgery. This was despite the fact that three of the five cases were graded to be of intermediate difficulty and one was graded to be of high difficulty. It is vital to highlight that an important confounding factor in the present study was that our team of surgeons had considerable experience with laparoscopic hepatectomy prior to embarking on robotic hepatectomy. The operating times for the five cases were relatively long; this could be due in part to the initial learning curve and the difficulty level of the cases. Nonetheless, it is important to emphasise that despite the long surgical times, none of the five patients experienced any major complications. We found that the superiority of the robot in performing fine suturing in tight spaces allowed for better control and haemostasis of bleeding vessels. We experienced this advantage in Case 2 (bile leakage from the segment V pedicle was oversewn) and Case 4 (bleeding from torn tributaries of the right hepatic vein was sutured). Other advantages of using the robot include the high-definition, three-dimensional visualisation, and the increased stability and dexterity of the robotic arms (which allowed for more precise dissection and transection of the liver, especially during transaction of the superior aspects of parenchymal transection). We were not able to assess the other reported benefits of robotic hepatectomy, such as the robot's performance in biliary-enteric anastomosis and porta hilar dissection, as these procedures were not performed in the present study.
Some of the technical limitations that we encountered with the robot were the lack of tactile feedback and the limited number of robotic instruments available for hepatectomy (as compared to conventional laparoscopy). The former can be partly overcome by improved three-dimensional visualisation and the presence of an expert surgeon at the bedside for surgical assistance, especially for suctioning. An example of the latter limitation is the lack of a laparoscopic CUSA for the robot. The laparoscopic CUSA is an instrument that we preferentially use for complex resections that require careful dissection around major vascular pedicles. As this instrument was not available with the robotic system, we had to rely on the assistant surgeon to drive this instrument in a robotic-laparoscopic hybrid technique for Cases 4 and 5.
In our opinion, robotic surgery is currently not in competition with, but rather, complementary to conventional laparoscopic hepatectomy. The application of robotic assistance in laparoscopic hepatectomy may be useful in expanding the indications for laparoscopic hepatectomy to more technically challenging procedures, and this would allow more patients to enjoy the benefits of minimally invasive surgery. Our initial experience with robotic hepatectomy demonstrated that the procedure is feasible and safe, even for hepatectomies that were graded to be of intermediate or high difficulty. Further studies involving larger patient cohorts are needed to determine whether robotic hepatectomy is superior and more cost-effective than conventional laparoscopic hepatectomy.
DISCLOSURE
Goh BK, Chan CY, Lee SY and Wong JS received travel support from Transmedic Pte Ltd, the local distributor of the da Vinci robotic system in Singapore.


