Abstract
A 39-year-old woman with end-stage renal disease, which was maintained on haemodialysis, developed secondary haemochromatosis after receiving blood transfusions and intravenous iron supplementation without sufficient serum ferritin concentration monitoring. The patient received intravenous deferoxamine three times a week, combined with high-dose recombinant human erythropoietin therapy and haemodialysis. After three months, improvements in biochemical indicators and iron overload were noted.
INTRODUCTION
Iron supplementation and/or blood transfusions combined with recombinant human erythropoietin (rHuEPO) injections are regularly used for correcting renal anaemia, which is a common complication of end-stage renal disease (ESRD). However, massive blood transfusions and inappropriate intravenous iron supplementation can lead to secondary haemochromatosis, resulting in damage to multiple organs. We herein report a rare case of secondary haemochromatosis in a haemodialysis patient.
CASE REPORT
On January 3, 2013, a 39-year-old Chinese woman on maintenance haemodialysis for ESRD was admitted with the chief complaint of bronze skin pigmentation. Two years prior to presentation, the patient was diagnosed with uraemia and renal anaemia at a local hospital, and had been receiving regular haemodialysis three times per week since diagnosis. To correct the anaemia, she had received blood transfusions of one unit of erythrocytes once per month and intravenous iron supplementation twice per week (200 mg/week) for two years, during which the patient’s skin became bronze in colour. Upon physical examination, she was found to have a temperature of 36.8°C, a heart rate of 80 bpm and blood pressure of 151/80 mmHg. Skin hyperpigmentation and distended neck veins were observed. The edge of her heart was found to extend to the lower left and a level III systolic murmur in the mitral area was heard. Her liver and spleen were palpable. There was obvious upper abdominal tenderness, especially in the right upper quadrant and spleen area.
Laboratory evaluation showed a haemoglobin level of 62 g/L. The patient’s total erythrocyte, leucocyte and platelet counts were 2.09 × 1012/L, 2.99 × 109/L and 54 × 109/L, respectively. Her serum creatinine level was 561 µmol/L and serum ferritin was 14,360 ng/mL, with transferrin saturation at 85.8%. Ultrasonography showed left ventricle hypertrophy, hepatomegaly and splenomegaly. Computed tomography (CT) revealed pulmonary hypertension, heart enlargement, manipulus, hydropericardium, and increased liver and spleen density (
Fig. 1
Abdominal CT image shows increased values in hepatic and spleen CT density: 104–106 HU in the liver and 80–94 HU in the spleen.
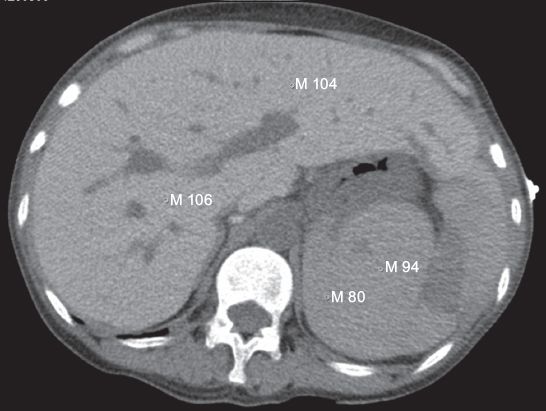
Fig. 2
T1- and T2-W MR images show marked hypointensity in the liver (arrows 1 and 4), spleen (arrows 3 and 6) and pancreas (arrows 2 and 5).
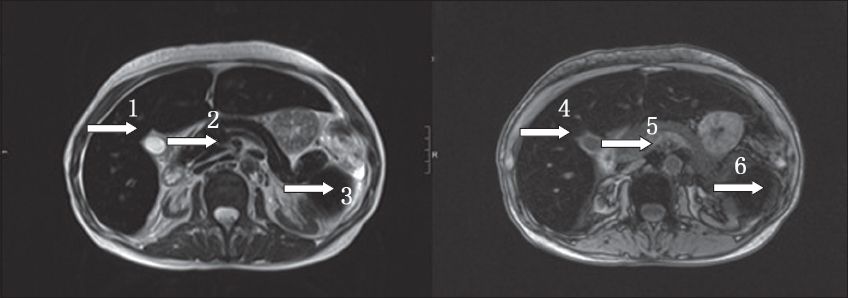
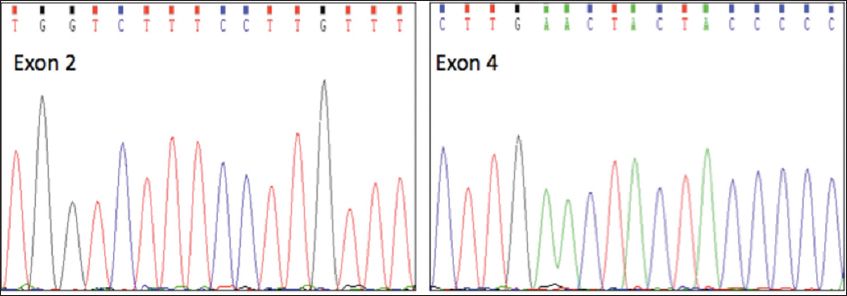
Fig. 3
DNA sequencing shows no mutation in exons 2 and 4, which contain the H63D and C282Y mutations, respectively.
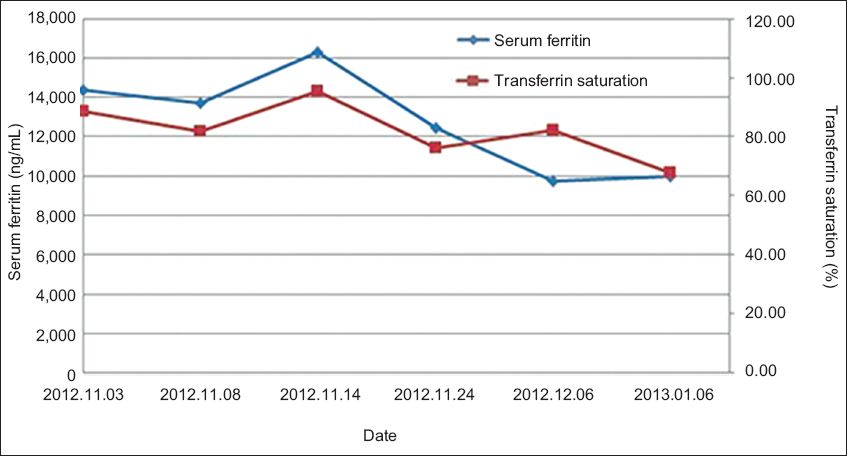
The patient’s treatments consisted of chelation therapy, intermittent haemodialysis and anaemia correction. She was continuously given 2,000 mg deferoxamine (50 mg/kg) intravenously over the course of eight hours, three times a week, prior to each episode of intermittent haemodialysis. No side effect was observed. rHuEPO was prescribed to improve anaemia. Over three months, the patient’s serum ferritin levels gradually decreased from 14,369 ng/mL to 9,720 ng/mL and transferrin saturation dropped from 85.8% to 67.1% (
Fig. 4
Graph shows changes in serum ferritin and transferrin saturation levels following treatment with deferoxamine and haemodialysis.
DISCUSSION
Haemochromatosis can be classified as either hereditary or secondary. Hereditary haemochromatosis, a genetic disorder causing excessive iron accumulation within the body, has a high morbidity among populations of northern European descent.(1) Secondary haemochromatosis, a possible consequence of chronic transfusion therapy for congenital or acquired anaemia, results in iron overloading and adversely affects functioning of the liver, heart and endocrine system. As each unit of erythrocyte concentrate contains 200–250 mg of iron, repeated blood transfusion therapy rapidly causes iron overloading.(2) Most haemochromatosis patients are asymptomatic. The main clinical manifestations observed include liver damage, skin bronzing or hyperpigmentation, diabetes mellitus, arthropathy, amenorrhoea, impotence, hypogonadism and cardiomyopathy.
Although it is recognised as the golden standard for the diagnosis of haemochromatosis, liver biopsy is an invasive procedure with potential risks. CT has low sensitivity and high specificity for measuring iron levels in the liver, and is helpful in confirming diagnoses. MR imaging is thus far the best noninvasive method for diagnosis of haemochromatosis, having both high sensitivity and specificity.(3) Early monitoring of sensitive markers plays an important role in avoiding damage to multiple organ systems. Generally, serum ferritin level and transferrin saturation are the most common and easily available parameters for monitoring iron levels in vivo. Although serum ferritin is an acute phase inflammatory protein and its level may rise when there is inflammation, infection and other disorders, the negative predictive value of combining the results of serum ferritin and transferrin saturation tests reaches 97%, which outweighs that of each index separately.(4)
Secondary haemochromatosis is rare in haemodialysis patients, but iron overload is common in ESRD populations. In 1978, Pitts et al reported iron overload (haemosiderosis) secondary to parenteral iron therapy in 24 patients on maintenance haemodialysis; however, haemochromatosis or organ dysfunction secondary to tissue iron deposits was not noted in any patient unless iron administration exceeded a total amount of 2.5 g.(5) A study of 172 ESRD haemodialysis patients with renal anaemia reported a prevalence of haemochromatosis with HFE gene mutation of as high as 41.3%.(6) However, there are no studies examining the morbidity of secondary haemochromatosis in haemodialysis patients.
Erythropoiesis-stimulating agents and iron supplementation are recommended by many guidelines for the treatment of anaemia associated with chronic kidney disease, and blood transfusion therapy is recommended as an acute treatment for anaemia.(7) Iron supplementation, particularly intravenous iron, has been shown to increase the effects of rHuEPO; however, it also increases the risk of iron overloading when subject to improper administration and poor monitoring of a patient’s iron status.
Treatment of haemochromatosis generally includes phlebotomy and chelation therapy. Phlebotomy, which removes excessive iron and maintains normal iron storage, remains the cornerstone therapy for hereditary haemochromatosis.(8) In patients with haemochromatosis and heart disease, anaemia or poor venous access, treatments with iron chelation agents are recommended.(9) Since being introduced into clinical practice in the early 1970s, deferoxamine has been established as an iron chelator, with numerous studies indicating that it has an obvious effect in lowering serum ferritin and liver iron levels.(10) In cases where deferoxamine therapy fails, high treatment costs and poor patient compliance rates are cited as being among the major reasons.(11) Deferoxamine shows a high penetration of hepatocytes and moderate penetration of the reticuloendothelial system and myocytes.(12) Biliary and blood excretion are the dual elimination mechanisms of deferoxamine, forming the final production in faeces and urine, respectively.(13) Generally, deferoxamine is injected subcutaneously for 8–12 hours per day at 40–60 mg/kg with the aid of an electronic pump.(14) A prospective study of 290 thalassaemia patients reported that a mean daily dose of deferoxamine at 51 mg/kg over one year resulted in a decrease of serum ferritin of 650 µg/L, which was more than what was observed when administering a dose of deferoxamine at 42 mg/kg.(12)
Haemodialysis allows low-molecular weight molecules of 5,000 Da or less to move freely across membranes.(15) Deferoxamine (560.68 Da) chelates ferric iron (56 Da) to create iron-bound deferoxamine, which can pass through the dialysis membrane. In ESRD patients with secondary haemochromatosis, chelation treatment should be combined with haemodialysis. Recently, Gumber et al reported a successful treatment of severe iron intoxication with deferoxamine and haemodialysis. Therefore, intermittent haemodialysis might aid in decreasing serum iron concentrations.(16)
In conclusion, it is important to follow guidelines to avoid secondary haemochromatosis caused by iatrogenic measures. Serum ferritin level and transferrin saturation are the most commonly used monitoring parameters of patients’ iron status, and MR imaging is the best noninvasive method of detection. Hereditary haemochromatosis should also be excluded through genetic testing. Early identification and diagnosis of haemochromatosis are important to avoid multiple organ damage. For ESRD patients with secondary haemochromatosis, iron chelation combined with haemodialysis is a feasible therapeutic regimen.


