Abstract
INTRODUCTION
Hypoglycaemia constitutes a significant barrier to achieving glycaemic control with insulin in both Type 1 (T1DM) and Type 2 diabetes mellitus (T2DM). The International Operations Hypoglycaemia Assessment Tool (IO HAT) study was designed to determine the incidence of hypoglycaemia in insulin-treated patients with T1DM and T2DM.
METHODS
The IO HAT study retrospectively and prospectively assessed the incidence of hypoglycaemia in patients with insulin-treated diabetes mellitus in nine countries. This sub-analysis included patients from Singapore with T1DM or T2DM who were aged ≥ 21 years and had completed two self-assessment questionnaires (SAQ1 and SAQ2).
RESULTS
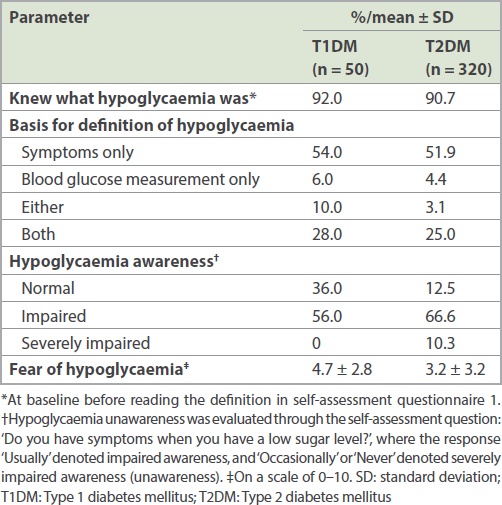
Of the 50 T1DM and 320 T2DM patients who completed the SAQ1, 39 T1DM and 265 T2DM patients completed SAQ2; 100% and 90.9%, respectively, experienced at least one hypoglycaemic event prospectively. The incidence rates of any hypoglycaemia were 49.5 events per patient-year (EPPY) and 16.1 EPPY for T1DM and T2DM patients, respectively, in the four-week prospective period. Hypoglycaemia rate did not differ in terms of glycated haemoglobin level. The vast majority of T1DM or T2DM patients (92.0% and 90.7%, respectively) knew the overall definition of hypoglycaemia before study participation, although over half of the patients (T1DM 54.0%, T2DM 51.9%) defined hypoglycaemia based only on symptoms.
CONCLUSION
High proportions of insulin-treated patients with diabetes mellitus in Singapore reported hypoglycaemic events prospectively, showing that they had underreported hypoglycaemic episodes retrospectively. Patient education can help in improving hypoglycaemia awareness and its management in the region.
INTRODUCTION
The prevalence of diabetes mellitus (DM) in Singapore is rapidly increasing.(1,2) The International Diabetes Federation’s 2015 report showed that there were 541,500 cases of DM in Singapore in 2015 alone.(3) In a report published by the Ministry of Health (MOH) Singapore in 2010, DM was found to be the second most important cause of disability-adjusted life years, a parameter to assess the burden of disease.(4)
One of the barriers to glycaemic control in both Type 1 DM (T1DM) and Type 2 DM (T2DM) is the potential hypoglycaemia that occurs with therapeutics.(5,6) Understanding the risk factors of hypoglycaemia in the specified population is the key to safer diabetic treatment for patients. Studies have identified various predictive factors associated with the risk of hypoglycaemia, including age, duration of DM, duration of insulin use, intensive glycaemic therapy, previous history of severe hypoglycaemia, renal impairment and alcohol consumption.(7,8)
In Singapore, the MOH has issued clinical practice guidelines for clinicians for the management of DM.(9) The guidelines recommend educating insulin-treated patients with DM regarding hypoglycaemia management and self-monitoring of blood glucose (SMBG). Clinicians also need to be vigilant with patients who have near-normal glycaemic levels, as they are at higher risk of hypoglycaemia.(9) Fear of hypoglycaemia is quite high in insulin-treated patients with DM and has a negative effect on the emotional state and well-being of the patient. This may, in turn, hinder optimal glycaemic control by preventing intensification of insulin therapy by physicians.(10,11) A local study in 2008 assessing the cost of DM and its related complications over five years showed that severe hypoglycaemia significantly increased healthcare costs by SGD 757 within the first year.(12) Additionally, the duration of hospitalisation is known to be higher in patients with DM who experience hypoglycaemia.(13) Nevertheless, aside from limited reports from clinical trials, real-world data on the evaluation of health service utilisation or hospitalisation related to hypoglycaemic events is lacking.(14) The seemingly high prevalence of hypoglycaemia and its associated economic burden necessitates a comprehensive reassessment of the real-world burden of hypoglycaemia in patients with DM in Singapore.
The non-interventional International Operations Hypoglycaemia Assessment Tool (IO HAT) study was designed to assess the incidence of hypoglycaemia in patients with insulin-treated DM in Bangladesh, Colombia, Egypt, Indonesia, the Philippines, Singapore, South Africa, Turkey and the United Arab Emirates.(15) The IO HAT study builds on findings from the global HAT (Hypoglycaemia Assessment Tool) study that was conducted in 24 countries.(16) In this sub-analysis, we assessed the incidence of hypoglycaemia retrospectively and prospectively among insulin-treated patients in Singapore with T1DM or T2DM.
METHODS
The IO HAT study was a non-interventional, multicentre, six-month retrospective and four-week prospective international survey on hypoglycaemia among insulin-treated patients with DM. It was conducted using two-part self-assessment questionnaires (SAQ1 and SAQ2) and a patient diary (PD) (
Box 1
International Operations Hypoglycaemia Assessment Tool study design:

The survey population comprised ambulatory and literate Singaporean patients with T1DM or T2DM who: were ≥ 21 years of age at baseline; were on insulin treatment (pre-mixed, short-acting and/or long-acting) for > 12 months; and had given informed consent before participating in the survey. Patients were enrolled randomly via consecutive sampling during a routinely scheduled clinical consultation with their healthcare provider at primary or secondary care centres in Singapore.
The primary endpoint of the survey was to evaluate the proportion of patients experiencing at least one hypoglycaemic event during the four-week prospective observational period. Secondary endpoints included determination of incidence rates (IRs) of all types of hypoglycaemia in the four weeks before (six months before baseline for severe hypoglycaemia) and four weeks after the baseline visit; the relationship between glycated haemoglobin (HbA1c) at baseline (HbA1c level < 7.0%, 7.0%–9.0% and > 9.0%), insulin regimen (short-acting, long-acting, pre-mix, and combination of short- and long-acting insulin) and hypoglycaemia, frequency of SMBG, patient knowledge and fear of hypoglycaemia; and the impact of hypoglycaemia on the healthcare system, work and/or studies.
The SAQ1 was used to record baseline demographics, DM-related complications and treatment information; evaluate knowledge of hypoglycaemia, hypoglycaemic unawareness and perceptions of hypoglycaemia; and record the history of severe hypoglycaemia over the previous six months and any (including nocturnal) hypoglycaemia over the previous four weeks. The SAQ2 was used to collect information on hypoglycaemia during the four weeks’ prospective period, and the effect of hypoglycaemia on productivity and healthcare utilisation during this time frame. Patients were also provided with PDs to record any hypoglycaemic events occurring in the four weeks after baseline and to assist recall.
Patients who returned any part of any SAQ or PD containing answers to any of the questions received were also included in the full analysis set. Patients’ knowledge of hypoglycaemia was assessed by checking if their definition was consistent with American Diabetes Association’s hypoglycaemia definition(19) and if they were aware of hypoglycaemia before reading the introduction provided in the informed consent form. Hypoglycaemia unawareness was evaluated with an SAQ question, ‘Do you have symptoms when you have a low sugar level?’, where the response ‘Usually’ denoted impaired awareness, and ‘Occasionally’ or ‘Never’ denoted severely impaired awareness (unawareness).(20) Fear of hypoglycaemia was rated by the patient on a scale of 0 (not afraid at all) to 10 (absolutely terrified).
Hypoglycaemia, as documented in the SAQ and PD, was classified as (a) non-severe hypoglycaemia: documented symptomatic (symptoms and blood glucose measurement ≤ 3.9 mmol/L [70 mg/dL]) and probable symptomatic (symptoms only) hypoglycaemia; (b) severe hypoglycaemia: an event requiring the assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions (based on the American Diabetes Association’s hypoglycaemia definition);(19) (c) nocturnal hypoglycaemia: an event occurring between midnight and 6.00 am; and (d) any hypoglycaemia: an aggregate index of all hypoglycaemic events from any of the categories.
The incidence of hypoglycaemia was compared in the retrospective and prospective periods using two-sided tests, in association with the DM type and insulin regimen used. Statistical significance was set at p < 0.05. For the primary endpoint, the proportion of patients who experienced at least one hypoglycaemic episode during the four-week prospective observational period among T1DM or T2DM was calculated together with the 95% confidence interval (CI). Incidence rates of hypoglycaemia were reported as events per patient-year (EPPY), calculated as the total number of events divided by total follow-up time in patient-years along with 95% CI. Relationship between HbA1c at baseline and the log-transformed number of events for patients experiencing hypoglycaemia were shown on a scatter plot (data not presented) with regression line and 95% CI. Continuous and categorical data were summarised using descriptive statistics and frequency tables (number of patients and percentage), respectively. Baseline data refers to data collected using the SAQ1, while follow-up data refers to data collected using the SAQ2 and, where applicable, PD.
RESULTS
A total of 370 patients (50 T1DM, 320 T2DM) were enrolled from study sites in Singapore and completed the SAQ1. Of these, 304 patients (39 T1DM, 265 T2DM) completed the SAQ2 and 307 patients (39 T1DM, 268 T2DM) completed the PD.
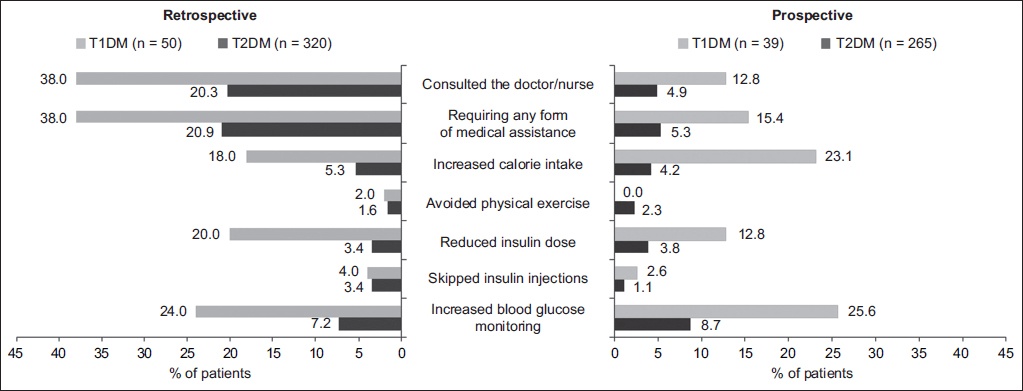
The baseline characteristics of the survey population are presented in
Table I
Patient baseline characteristics.
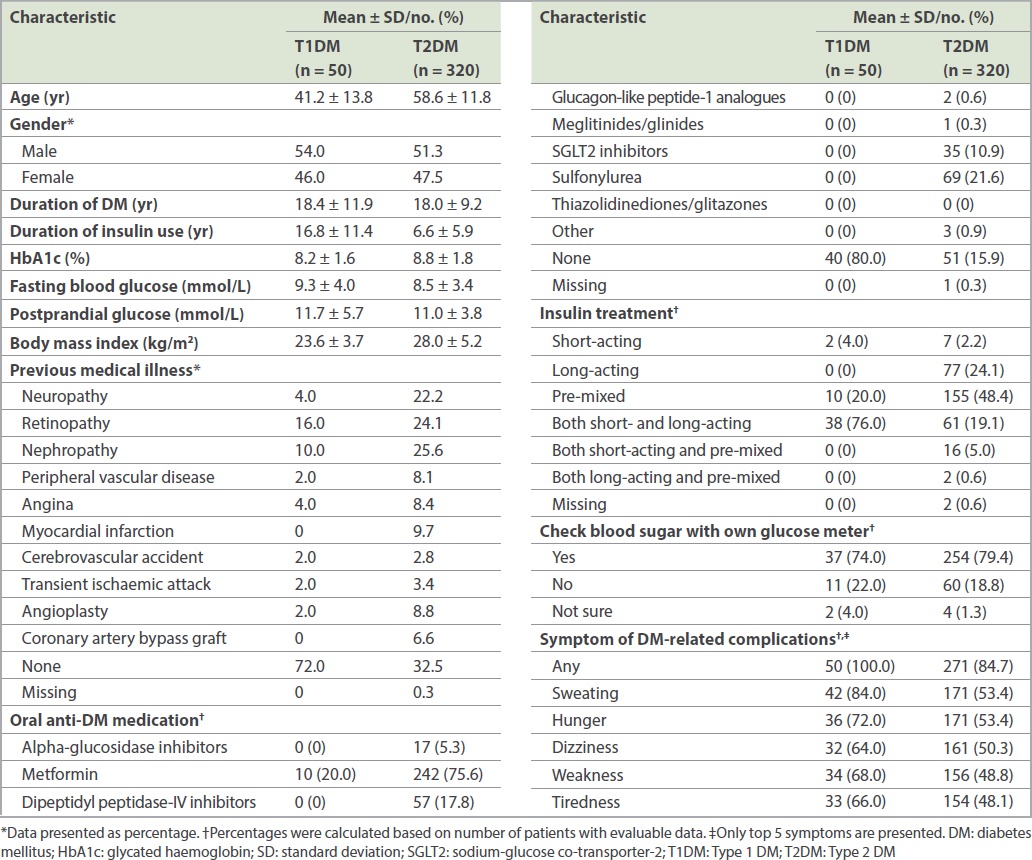
In the four-week prospective period, the proportion of patients reporting at least one hypoglycaemic event was 100.0% (95% CI 91.0%–100.0%) with T1DM and 90.9% (95% CI 86.7%–94.1%) with T2DM, while in the four-week retrospective period, 53.1% (95% CI 38.3%–67.5%) of patients with T1DM and 20.4% (95% CI 16.1%–25.3%) of patients with T2DM experienced at least one hypoglycaemic event. In patients with T1DM, the estimated IR of any hypoglycaemia was significantly higher in the prospective period than in the retrospective period (49.5 [95% CI 41.9–58.2] EPPY vs. 18.4 [95% CI 14.3–23.2] EPPY, p < 0.001) (
Fig 1
Bar graphs show retrospective and prospective hypoglycaemic rates in patients with (a) Type 1 and (b) Type 2 diabetes mellitus. For any or nocturnal hypoglycaemia, data was based on a four-week period for both analyses. For severe hypoglycaemia, retrospective data was based on a six-month period and prospective data was based on a four-week period. RR: rate ratio
In nocturnal hypoglycaemia, the proportion of patients reporting a hypoglycaemic event in the retrospective period was 37.0% (95% CI 23.2%–52.5%) and 9.2% (95% CI 6.3%–13.0%) in T1DM and T2DM, respectively, whereas 38.5% (95% CI 23.4%–55.4%) with T1DM and 7.6% (95% CI 4.7%–11.5%) with T2DM experienced at least one hypoglycaemic event in the prospective period. In the patients with T1DM, there was no significant difference in the estimated IR of nocturnal hypoglycaemia between the prospective period and the retrospective period (11.0 [95% CI 7.6–15.5] EPPY vs. 7.9 [95% CI 5.3–11.5] EPPY, p = 0.292;
In T1DM, 35.4% (95% CI 22.2%–50.5%) of patients in the six-month retrospective period and 41.0% (95% CI 25.6%–57.9%) of patients in the four-week prospective period experienced a severe hypoglycaemic event. The IR of severe hypoglycaemia was significantly higher in the prospective period compared to the retrospective period (8.7 [95% CI 5.7–12.7] EPPY vs. 1.0 [95% CI 0.7–1.6] EPPY, p < 0.001) (
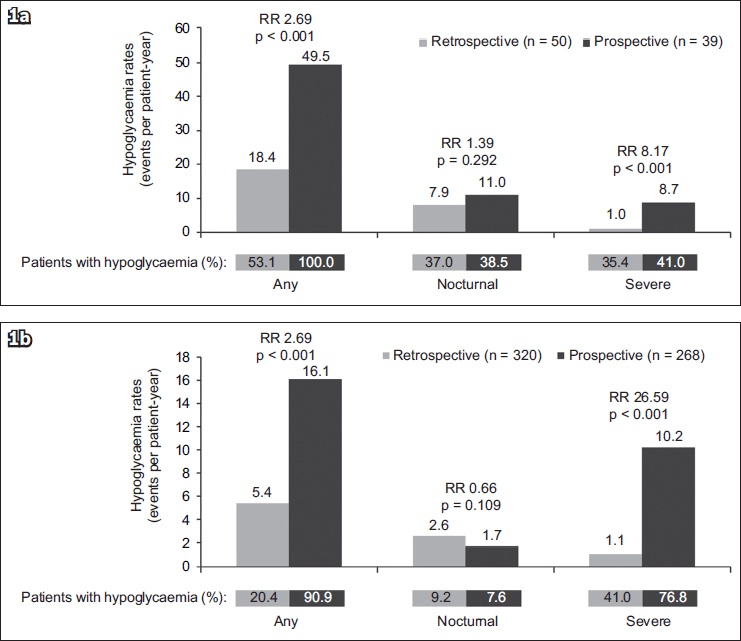
The impact of hypoglycaemic events on the medical system during the retrospective and prospective periods is presented in
Table II
Impact of hypoglycaemic events on medical system and work/studies.
A total of 82.0% patients with T1DM and 47.8% patients with T2DM were students or full- or part-time employees. In the retrospective period, more patients with T1DM than those with T2DM experienced hypoglycaemic events that resulted in absence from work or studies (7.3% vs. 2.6%, respectively), late arrival to work or studies (7.3% vs. 2.0%, respectively), or early departure from work or studies (4.9% vs. 2.0%, respectively;
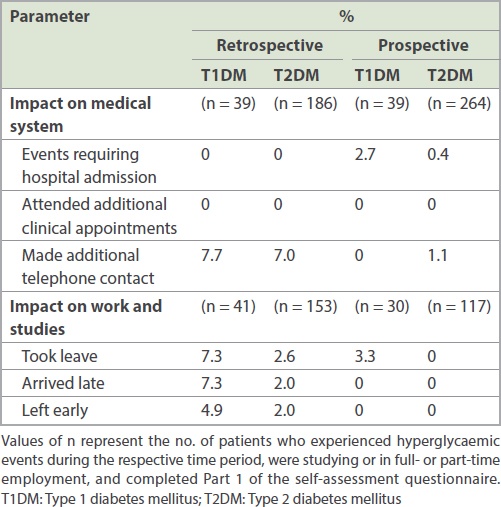
Different actions were taken by patients in response to hypoglycaemic events (
Fig 2
Graph shows patient actions resulting from hypoglycaemia, based on the proportion of patients who responded ‘Yes’ to each question. T1DM: Type 1 diabetes mellitus; T2DM: Type 2 diabetes mellitus
Patients’ perspectives on hypoglycaemia are provided in
Table III
Patient perspectives on hypoglycaemia.
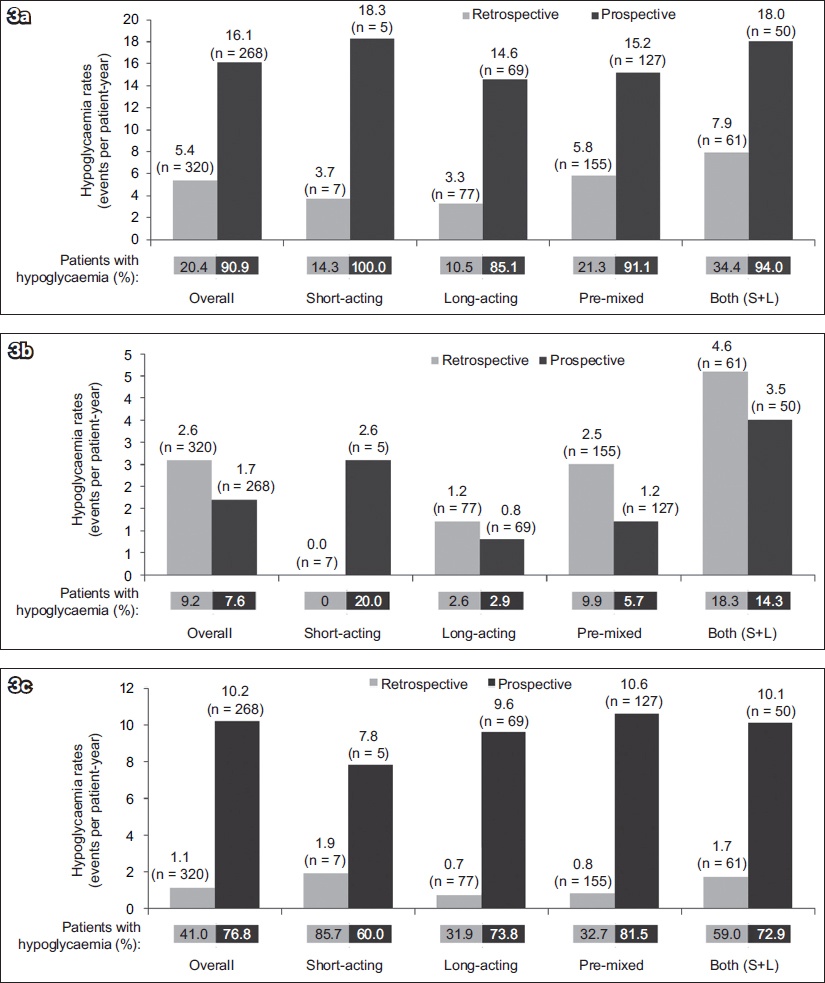
For patients with T1DM, the overall IRs of any, nocturnal and severe hypoglycaemia were higher in the prospective period. The highest IRs of any hypoglycaemia were observed with the use of a combination of short- and long-acting insulin regimens (57.4 EPPY) in the prospective period. The IRs of nocturnal hypoglycaemia were also highest for patients using a combination of short- and long-acting insulin regimens (11.7 EPPY) in the prospective period. The IRs for severe hypoglycaemia were the highest for short-acting insulin regimens (13.0 EPPY) in the prospective period and lowest for the combined short- and long-acting insulin regimens (1.1 EPPY) in the retrospective period. For patients with T2DM, the proportion of participants and estimated IRs for all types of hypoglycaemia by insulin regimen are shown in
Fig 3
Bar graphs show hypoglycaemia incidence and event rate in patients with Type 2 diabetes mellitus, by insulin regimen: (a) any hypoglycaemia, (b) nocturnal hypoglycaemia and (c) severe hypoglycaemia. For any or nocturnal hypoglycaemia, data was based on a four-week period for both analyses. For severe hypoglycaemia, retrospective data was based on a six-month period and prospective data was based on a four-week period. S+L: short-acting and long-acting insulin
In this study, no correlation was seen between the proportion of patients with hypoglycaemia and baseline HbA1c in the retrospective and prospective periods. For patients with T1DM, in the four-week retrospective period, the majority (88.9%) of any hypoglycaemic events were experienced by patients in the good glycaemic control group (HbA1c < 7.0%), but the proportion of patients experiencing any hypoglycaemic events was higher in patients with poorly controlled glycaemia (HbA1c > 9.0%) than in those with suboptimal glycaemic control (HbA1c 7.0%–9.0%) (58.3% vs. 39.3%, respectively). For patients with T2DM, the proportions of patients in the three baseline HbA1c groups who had any hypoglycaemia were similar in the four-week retrospective period (16.0%–27.3%).
DISCUSSION
This sub-analysis examined the prevalence of hypoglycaemic events in insulin-treated DM patients in the Singapore cohort of the IO HAT study. This dataset also provides information about knowledge and awareness of hypoglycaemia and its impact on the daily life of patients in Singapore.
The results showed that a higher percentage of patients reported hypoglycaemia in the prospective period compared to the retrospective period. This could be due to recall bias, since it is understandably more difficult to remember events retrospectively than prospectively, resulting in underreporting of events. In the prospective period, in addition to using the PD, patients are more likely to take note of possible hypoglycaemic symptoms. This recall bias was more pronounced for patients with severe hypoglycaemia, as they were required to retrospectively recall an event from the past six months; in contrast, the period of recall was just four weeks for any hypoglycaemia. The higher incidence of hypoglycaemia reporting in the prospective period could also be due to the SAQs and PDs, which served as tools of documentation and knowledge reinforcement.
The IRs of any and severe hypoglycaemia were significantly lower in the retrospective period compared to the prospective period, showing that although patients had good knowledge of hypoglycaemia at baseline, this did not translate to accurate reporting of hypoglycaemic events. In practice, healthcare providers ought to be aware that their patients are likely to underreport hypoglycaemia based on recall alone. It would be a good practice to encourage patients to keep a diary of hypoglycaemic events for better documentation of events to facilitate further prevention and management.
Notably, nocturnal hypoglycaemia did not significantly differ between the assessment periods for patients with T1DM or T2DM. This might be because some of the patients had hypoglycaemia unawareness and thus reported less nocturnal hypoglycaemia than expected. Also, nocturnal hypoglycaemia (from midnight to 6.00 am) may go unnoticed and or unreported, as the events could occur during sleep hours, when the intensity and recognisability of counter regulatory responses tend to be diminished.(21) A slightly higher percentage of patients reported hypoglycaemia in the retrospective period than the prospective period (2.6% vs. 1.7%) in the T2DM group. Patients may have reported higher incidences of nocturnal hypoglycaemia retrospectively when they were asked to recall, whereas having a clear definition of nocturnal hypoglycaemia in the prospective period allowed more accurate reporting during this period. Another perspective would be that nocturnal events may have impacted patients more, hence there was less recall bias.
Overall, for patients with T2DM, the rates of hypoglycaemia did not differ with the type of insulin regimen used. In T1DM, a high proportion of patients (76%, n = 38) were on a combination of short- and long-acting insulins, followed by pre-mixed insulin (20%, n = 10) and short-acting insulin (4%, n = 2;
Similar to findings from the primary IO HAT study,(15) results from this cohort showed that the rate of any hypoglycaemic event was independent of HbA1c levels at baseline. Therefore, hypoglycaemia can also occur in patients with higher HbA1c levels. These results are in line with the findings from a recently published study, where HbA1c levels were not associated with risk of hypoglycaemia in an older population with T2DM who were on insulin therapy.(24) Thus, it is important for healthcare providers to proactively screen patients for hypoglycaemia events regardless of their HbA1c reading.
Patients in Singapore with T1DM or T2DM had good knowledge and were capable of identifying hypoglycaemia. Despite this, the knowledge was not transferred to actual practice; 50% of the study population defined hypoglycaemia based on symptoms alone and only a quarter confirmed the symptoms with a blood glucose check. The majority of patients had impaired hypoglycaemia awareness, which indicates the importance of SMBG. SMBG can be a valuable focus as we enhance patient knowledge of the management of hypoglycaemia.
Goh et al, in their review of the use of insulin and risk of hypoglycaemia in the Southeast Asian population, reported that hypoglycaemic events result in increased medical complications, medical expenditure, loss of productivity and trauma in patients with DM.(25) The fear of hypoglycaemia in such patients adversely affects glycaemic control by preventing intensification of insulin therapy or, in some cases, even resulting in discontinuation of insulin therapy.(25,26) Results from the current study also demonstrate the substantial impact of hypoglycaemia on the healthcare system, work and productivity. Unfortunately, very few patients with T2DM took action in response to hypoglycaemic events, highlighting the need for patient counselling on recognition and management of hypoglycaemia.
Similar to the global HAT study, this sub-analysis was limited by its observational nature and short prospective duration. Recall bias associated with retrospective periods may be one of the limitations preventing direct comparison of prospective and retrospective periods. Potentially, the incidence of hypoglycaemia may be under- or overestimated due to self-reporting of data in the PD and SAQ2. Data obtained from the current study can pave the way for future research on managing hypoglycaemia in patients in Singapore, as it shows that patient education on hypoglycaemia and other factors such as regular SMBG and better tailoring of insulin treatment for patients with DM can help in minimising the risk of hypoglycaemia.
In conclusion, this study has shown the magnitude of hypoglycaemia among insulin-treated patients with T1DM and T2DM in Singapore. A high proportion of patients reported hypoglycaemic events with the use of questionnaires and PDs during the prospective period. However, healthcare providers ought to be aware that hypoglycaemic events can be underreported in insulin-treated patients locally. The majority of the patients are unlikely to confirm hypoglycaemic symptoms with blood glucose checks, and most T2DM patients would not take action. There is a need to focus on these areas when educating patients in managing hypoglycaemia. It is also important to explore ways to improve documentation of hypoglycaemia events as they occur. Accurate reporting and appropriate management of hypoglycaemia can improve the overall well-being of the patient.


