Abstract
Multiple myeloma (MM) is an incurable plasma cell neoplasm with an incidence of 100 patients per year in Singapore. Major advances have been made in the diagnosis, risk stratification and treatment of MM in the recent past. The reclassification of a subset of patients with smouldering MM, based on high-risk biomarkers, and the development of the revised international staging system are among the key new developments in diagnosis and staging. The use of novel agent-based treatment has resulted in significant improvements in the survival and quality of life of many patients with MM. Determining the optimal use of proteasome inhibitors, immunomodulators and, more recently, monoclonal antibodies is an area of ongoing investigation. In this guideline, we aim to provide an overview of the management of MM, incorporating the latest developments in diagnosis and treatment.
INTRODUCTION
Approximately 100 people per year are diagnosed with multiple myeloma (MM) in Singapore. Recent therapeutic advances with novel agents have changed the disease landscape, and while MM was once largely untreatable, patients now have a higher likelihood of entering remission with prolonged survival. These guidelines were developed by the Singapore Myeloma Study Group (SMSG) to provide evidence-based recommendations for the diagnosis and management of MM in the local setting. This guideline is not intended to be prescriptive and should be used in conjunction with physicians’ clinical judgement. The guideline is divided into five sections:
I. Diagnosis, staging and risk stratification
II. Supportive care
III. Management of transplant-eligible patients
IV. Drug toxicity and dose adjustments
METHODS
The SMSG performed a review of the key literature until 31 December 2015. These included MEDLINE, Cochrane Library and major meeting reports from the American Society of Hematology, American Society of Clinical Oncology, European Society of Hematology and the International Myeloma Workshop. Key recommendations from the International Myeloma Working Group (IMWG) and British Committee for Standards in Haematology have also been incorporated. These were summarised into a draft that the SMSG revised. It also proposed recommendations in situations where there was insufficient published data. We suggest the IMWG guidelines as a reference for readers looking for more details on specific aspects of the management of MM.
I. DIAGNOSIS, STAGING AND RISK STRATIFICATION
Background
The diagnosis and staging of MM is a rapidly evolving field. The definition of MM in previous IMWG consensus statements required the presence of organ damage, specifically hypercalcaemia, renal impairment, anaemia and bone lesions, commonly recognised by the acronym CRAB.(1) Monoclonal gammopathy of undetermined significance (MGUS) and smouldering multiple myeloma (SMM) are defined by the presence of specific levels of monoclonal protein and clonal bone marrow plasma cells, in the absence of organ involvement, and the majority of these patients progress to develop MM at varying rates.(1)
The traditional approach to management of these patients is to withhold treatment until the onset of organ involvement, given the absence of evidence that early treatment impacts outcome.(2) The treatment options for MM have improved significantly over the last ten years.(3) Recent data showed that a subset of patients who lacked organ damage and were classified as SMM according to the previous IMWG criteria have active disease that may benefit from treatment.(4,5) The IMWG has updated its definition of MM to take these changes into account.(6) The following are the key changes made in the 2014 IMWG consensus on diagnosis of MM:
- The presence of ≥ 10% monoclonal plasma cells in the marrow is a requirement for the diagnosis of MM.
- The presence of a monoclonal protein in the serum or urine is not mandatory to make a diagnosis of MM.
- In the absence of CRAB features, the presence of at least one of three high-risk biomarkers (see next section) is sufficient to establish a diagnosis of MM.
Definition of symptomatic multiple myeloma
According to the IMWG 2014 consensus, a diagnosis of MM can be made in the presence of monoclonal plasma cells in the bone marrow (≥ 10%), or biopsy-proven bony or extramedullary plasmacytoma, and any one or more of the following myeloma-defining events:
1. End-organ damage attributable to the underlying plasma cell proliferative disorder:
-
Calcium elevation (> 2.75 mmol/L or > 0.25 mmol/L above upper limit of the normal)
-
Renal dysfunction (creatinine clearance < 40 mL/min or serum creatinine > 177µmol/L)
-
Anaemia (haemoglobin [Hb] < 10 g/dL or > 2 g/dL below lower limit of the normal)
-
Bone disease (≥ 1 osteolytic lesions on skeletal radiography, computed tomography [CT] or magnetic resonance [MR] imaging)
2. Any one or more of the following features (biomarkers of high risk):
-
Clonal bone marrow plasma cell percentage ≥ 60%
-
Involved/uninvolved serum free light chain (SFLC) ratio ≥ 100
-
More than one focal lesion on magnetic resonance imaging studies
Recommendations
1. Screening for monoclonal gammopathy
Although the presence of a monoclonal protein is not mandatory for the diagnosis of MM, approximately 97% of patients with MM have a monoclonal protein.(6) The finding of a monoclonal protein is also a frequent cause of referrals from other medical specialists who screen patients for monoclonal gammopathies. Serum protein electrophoresis (SPEP) is often used as a screening test to identify a monoclonal protein. Serum immunofixation (IFE) is, however, mandatory to confirm monoclonality. SFLC is important in the diagnosis of patients with light chain MM who may have CRAB features but no evidence of a monoclonal heavy or light chain on SPEP and IFE. SLFC is also relevant for the prognostication of patients with MGUS and to identify patients with ‘high-risk SMM’, which would now be classified as MM. Furthermore, SFLC plays an important role in the diagnosis of light chain amyloidosis.(7) We therefore recommend that SPEP, IFE and SFLC be used as screening tests for a monoclonal protein (
Box 1
Recommended investigations – screening for monoclonal gammopathy

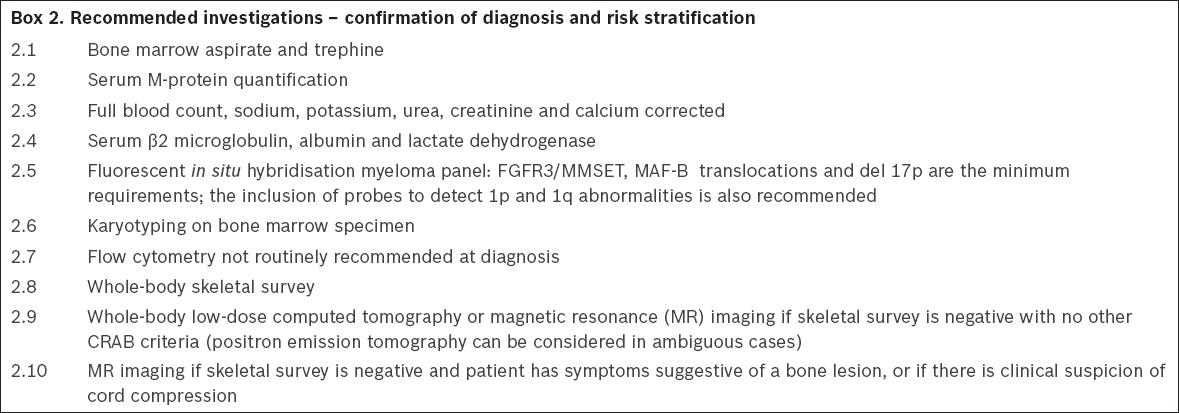
2. Investigations for confirmation of diagnosis and risk stratification
Bone marrow aspiration and trephine biopsy (BMAT) are mandatory for the diagnosis of MM. Immunohistochemical assessment of light chain restriction on the trephine specimen is recommended to confirm plasma cell clonality.(6) BMAT is especially important in the 3% of patients with nonsecretory MM who present with CRAB features and no evidence of a monoclonal protein on the screening investigations described above. A full blood count (FBC), serum creatinine levels and calcium levels are essential to confirm the presence of anaemia, renal impairment and hypercalcaemia. Quantification of the serum M-protein by densitometry is recommended at diagnosis, as this provides a baseline for the assessment of treatment response.(8)
A whole-body skeletal survey is recommended as the first-line investigation for lytic skeletal lesions. If the skeletal survey shows no lytic lesions, a whole-body low-dose CT (WBLDCT) or MR imaging is recommended if the patient has no other CRAB features.(9) Whole-body MR imaging or WBLDCT is also recommended if the skeletal survey is negative and the patient has symptoms that suggest bone lesions. MR imaging of the spine is indicated if there is a clinical suspicion of spinal cord compression. Whole-body MR imaging is also recommended for all patients diagnosed with SMM, as the finding of a lesion on MR imaging will lead to the diagnosis being reclassified as MM. Positron emission tomography (PET) should be considered in the event that a definitive diagnosis of bone lesions is not possible based on skeletal survey (CT or MR imaging).(6) MR imaging and PET-CT are also important tools for distinguishing truly solitary bone plasmacytoma from MM by identifying additional occult bone lesions (
Box 2
Recommended investigations – confirmation of diagnosis and risk stratification
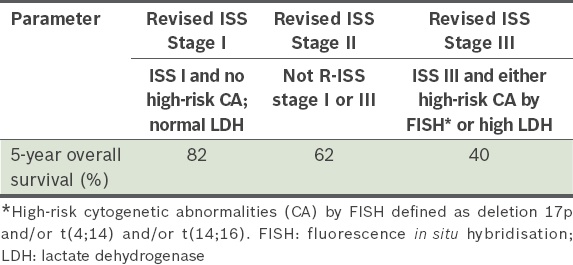
Risk stratification of MM during the last decade has been based on the international staging system (ISS;
Table 1
International Staging System.

Table 2
Revised International Staging System (R-ISS).
3. Pre-treatment evaluation
Pre-treatment evaluation points 1–9, as suggested below, are recommended for all patients with newly diagnosed MM. Point 10, the protocol for financial assessment and assistance, may vary between institutions.
-
Height, weight and body surface area to be recorded
-
Urine human chorionic gonadotropin test for females of childbearing age
-
Liver function tests
-
Viral screen: hepatitis B surface antigen, anti-hepatitis B core total, anti-hepatitis C virus and HIV serology
-
Glucose-6-phosphate dehydrogenase quantification
-
Contraception (use during therapy and for two years after treatment is advised)
-
Dental review (pre-bisphosphonate): patients who have undergone dental extraction should have a two-week rest period prior to commencement of bisphosphonate(12)
-
25-hydroxy vitamin D level
-
Consent for chemotherapy and counselling about treatment regimen and side effects (acute and long-term)
-
Financial assessment and referral to social worker for bortezomib and lenalidomide
4. Conclusion
Our knowledge of the biology of MM has increased rapidly over the last decade. Therefore, the diagnosis and prognostication of MM has evolved significantly, with a number of clinical and genetic parameters proving to be of prognostic use. It is noteworthy, however, that selected investigations (such as those used in the R-ISS) can provide accurate prognostication at a reasonable cost. There is little doubt that the diagnosis and risk stratification of MM will further evolve in the near future, allowing for more targeted and risk-adapted therapeutic approaches.(13)
II. SUPPORTIVE CARE
Introduction
Holistic care for patients with MM goes beyond offering the best available anti-myeloma treatment options to patients. While MM is still incurable, novel therapies have vastly improved the response rates and options available to patients following relapses, hence improving survival.(12) With improved lifespans, patients with MM have become more vulnerable to the cumulative toxicity of treatments. The symptom burden in this group of patients may not necessarily be improved with the introduction of more anti-myeloma treatment options. The important role of supportive care, to ensure that these patients remain minimally affected by the complications of disease and treatment, should not be overlooked, for it ensures that their quality of life is not compromised.
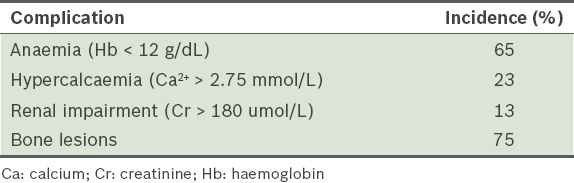
Complications related to multiple myeloma
The incidence of MM-related organ and tissue involvement at initial presentation is summarised in
Hypercalcaemia
Osteoclast-mediated bone destruction in MM may lead to hypercalcaemia. It has a broad spectrum of clinical manifestations ranging from polydipsia, polyuria and abdominal pain to renal and even neurological deficits, including coma and obtundation.(17) When other causes of hypercalcaemia have been excluded, definitive treatment for MM should be undertaken without delay. Supportive therapy should also be commenced while awaiting a response to the definitive therapy.
Hydration with intravenous normal saline is usually adequate for mild hypercalcaemia (Ca2+ = 2.6–2.9 mmol/L). For moderate to severe hypercalcaemia (Ca2+ > 2.9 mmol/L), bisphosphonates should be given in addition to hydration. In the treatment of malignancy-related hypercalcaemia, intravenous zoledronic acid 4 mg was found to be superior to intravenous pamidronate in resolving hypercalcaemia.(18) Close monitoring of fluid balance and expectant diuresis should also be considered.(15)
Renal complications
Renal impairment in MM patients occurs as a result of light chain medicated damage of renal tubules, together with a combination of hypercalcaemia, infections and use of nephrotoxic agents.(19) In patients presenting with renal impairment, there is a pressing need to curtail further worsening of renal function and possibly reverse renal insults, in order to avoid the need for long-term renal replacement therapy. To prevent further worsening of renal function, hydration should be optimised and nephrotoxic agents avoided. Early institution of anti-myeloma treatment has the largest impact on reversal of renal damage, as it can reverse myeloma-related renal complications in up to 50% of patients.(20) This hinges upon attaining an accurate diagnosis and degree of light chain involvement as soon as possible. Bortezomib with high-dose dexamethasone has been found to be effective in this setting.(20)
At the time of publication, there is insufficient evidence to support the role of plasma exchange or high cut-off dialysis in patients with suspected light chain cast nephropathy. A meta-analysis of 147 patients with MM and renal failure suggested improved renal outcome in patients treated with chemotherapy and plasmapheresis, rather than chemotherapy alone.(21) Small cohort studies reported sustained renal recovery and dialysis independence in 75% of patients with renal impairment who were placed on high cut-off dialysis.(22) However, larger-scale studies are required to provide conclusive guidelines on the use of plasmapheresis or high cut-off dialysis in MM patients with severe renal impairment.
Anaemia
Anaemia is a frequent complication in MM and may significantly impair patients’ quality of life.(23,24) After exclusion of haematinic deficiency or blood loss, patients with persistent Hb < 10 g/dL should receive treatment for anaemia attributable to myeloma. A therapeutic trial of an erythropoiesis-stimulating agent may be administered, with the aim to increase Hb levels without exceeding 12 g/dL. Subcutaneous erythropoietin alfa 40,000 units or erythropoietin beta 30,000 units per week may be used at the start of the therapy.(15) Effective treatment of anaemia with erythropoietin effectively decreases transfusion requirements and improves quality of life.(23) Erythropoietin can be stopped if no response is observed after 6–8 weeks.
Management of bone disease and related complications
MM bone disease often results in pain, pathologic fractures and spinal cord compression.(14) Long bone fractures require stabilisation and consideration of subsequent radiotherapy. Local radiotherapy with 8 Gy in a single fraction has been shown to be useful for pain relief.(25) An orthopaedic opinion should be sought to consider pre-emptive surgery for any large lytic lesion that may potentially cause instability. If there is any clinical suspicion of spinal cord compression, urgent MR imaging of the spine should be performed and orthopaedic surgeons consulted regarding the need for immediate surgical intervention. Dexamethasone 40 mg daily should be commenced in addition to spinal nursing. Adjunctive radiotherapy may be employed to control tumour growth and prevent irreversible neurological damage.(26)
All patients with symptomatic MM, regardless of the presence of bone lesions, should be treated with a bisphosphonate, the first choice being zoledronic acid. Beyond its role in bone health, zoledronic acid has been shown to have anti-cancer activity in myeloma, with improvements to overall survival seen in patients on treatment.(27) Pamidronate is an option in patients with a creatinine clearance of < 30 mL/min. A dose of 30 mg monthly is suggested. The minimum duration of bisphosphonate therapy is two years, on the condition that a very good partial response (VGPR) or complete response (CR) is achieved. Bisphosphonates should be restarted at the time of relapse.(12)
Denosumab, a human monoclonal antibody against the receptor activator of nuclear factor kappa-B ligand, has been demonstrated to reduce bone-related events in patients as effectively as zoledronic acid.(28) Bisphosphonate, but not denosumab, deposits in bone with a long half-life, which may make a difference in long-term efficacy as well as adverse effects.(29) Randomised trials comparing denosumab with zoledronic acid in MM are still in progress. Denosumab is, therefore, not recommended outside the context of a clinical trial at this point.
We recommend the measurement of 25-hydroxy vitamin D levels in all patients at diagnosis. Vitamin D and calcium replacement is indicated in patients with vitamin D deficiency or those on bisphosphonate treatment. It is contraindicated in patients with hypercalcaemia. The recommended daily dose for replacement is calcium 1,500 mg and vitamin D 1,000 IU.(30)
Infective complications
The combination of disease-related hypogammaglobulinaemia and treatment-related immunosuppression increases susceptibility to and severity of infections in MM patients.(15) Antiviral prophylaxis using acyclovir is recommended for patients receiving proteasome inhibitors (PI), and anti-Pneumocystis jiroveci pneumonia prophylaxis is recommended for patients receiving high-dose steroids.(31,32) The routine use of antibacterial and antifungal prophylaxis in MM patients cannot be recommended at the time of publication.
Prophylactic intravenous immunoglobulin may also be considered for patients in the plateau phase with hypogammaglobulinaemia and recurrent bacterial infections. We recommend intravenous immunoglobulin 0.4 g/kg monthly for six months in patients with > 2 significant infective episodes per year.(33) Prophylactic antiviral therapy for hepatitis B carriers and management of neutropenic fever should be carried out according to institutional protocols.
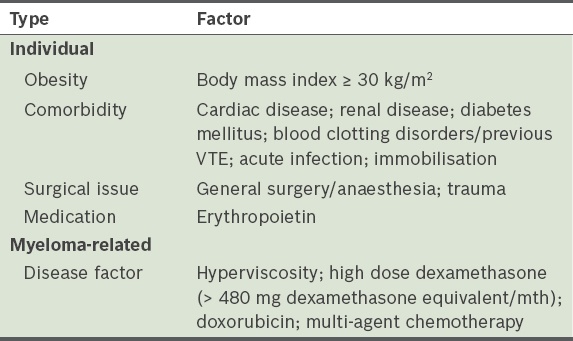
Thrombotic complications
Although patients with active malignancy have a higher risk of thromboembolic complications, current guidelines do not advocate routine thromboprophylaxis for patients with malignancy.(34) The use of immunomodulators, such as thalidomide and lenalidomide, increases the risk of venous thromboembolism in patients with MM, especially when used in combination with steroids or chemotherapy.(35,36) A meta-analysis performed on the thromboembolic risks of thalidomide and lenalidomide in MM patients demonstrated that the highest risk occurs in patients with newly diagnosed MM receiving thalidomide and dexamethasone without thromboprophylaxis. The three-month venous thromboembolism risk is 12% in this group. In other MM patients treated with thalidomide or lenalidomide, the venous thromboembolism risk was 3%–5%.(37)
There are no randomised trials comparing the treatment outcome of MM patients on thalidomide or lenalidomide with and without thromboprophylaxis. Moreover, there are insufficient observations regarding the risk of major bleeding on thromboprophylaxis. There are also no head-to-head comparisons between the various forms of anticoagulants (low-molecular-weight heparin vs. aspirin vs. target-specific oral anticoagulants). Some guidelines are based on extrapolations from other risk groups; hence, there is no available evidence-based guideline on the use of thromboprophylaxis in patients with MM who are treated with thalidomide and lenalidomide. While the IMWG recommends risk assessment, this approach has not been validated. It has been suggested that the use of prophylaxis may confer decreased risk, but no clear benefit has been proven.
The SMSG proposes the following recommendation with reference to the risk assessment model suggested by the IMWG (
-
For all newly diagnosed MM patients treated with thalidomide or lenalidomide, consider aspirin prophylaxis.
-
For all MM patients treated with thalidomide or lenalidomide in combination with steroids or chemotherapy, consider aspirin prophylaxis.
-
For all MM patients on thalidomide or lenalidomide with ≥ 2 other risk factors, consider low-molecular-weight heparin at prophylactic dose.
It is noteworthy that the incidence of venous thromboembolism in Asian MM patients on thalidomide and lenalidomide may be less than that of their western counterparts.(38,39) These recommendations should, therefore, be considered in this context, and the risk and benefit of anticoagulation assessed on a case-by-case basis.
Conclusion
Care of the patient with MM requires attention to factors beyond disease status. Attention to disease and treatment-related complications, as well as collaboration among primary care physicians, other specialists and haematologists, will ensure holistic patient care and maximise the benefits from treatment. Our recommendations for supportive care in MM are summarised in
Table 5
Summary of recommendations for supportive care of patients with multiple myeloma.

III. MANAGEMENT OF TRANSPLANT-ELIGIBLE PATIENTS
Background
Approximately 35% of patients with MM are below 65 years of age.(40) High-dose therapy followed by autologous stem cell transplantation (ASCT) has been shown to prolong survival in both the pre-novel agent and novel agent eras.(41,42) In this consensus statement, we summarise the evidence for treatment of transplant-eligible patients with MM and provide recommendations on the optimal treatment options.
Definition of transplant eligibility
Patients below 65 years of age with an acceptable comorbidity profile and performance status are considered transplant-eligible.(43) Selected patients aged above 65 years with good performance status and minimal comorbidities may be considered for reduced-intensity transplant.(44,45)
Indications for treatment
Clinical features defining symptomatic MM and/or one or more of the three high-risk biomarkers in patients who would previously have been classified as SMM are considered treatment indications.(5,6) Clinical features defining symptomatic MM and high-risk biomarkers are described in detail in Section I.
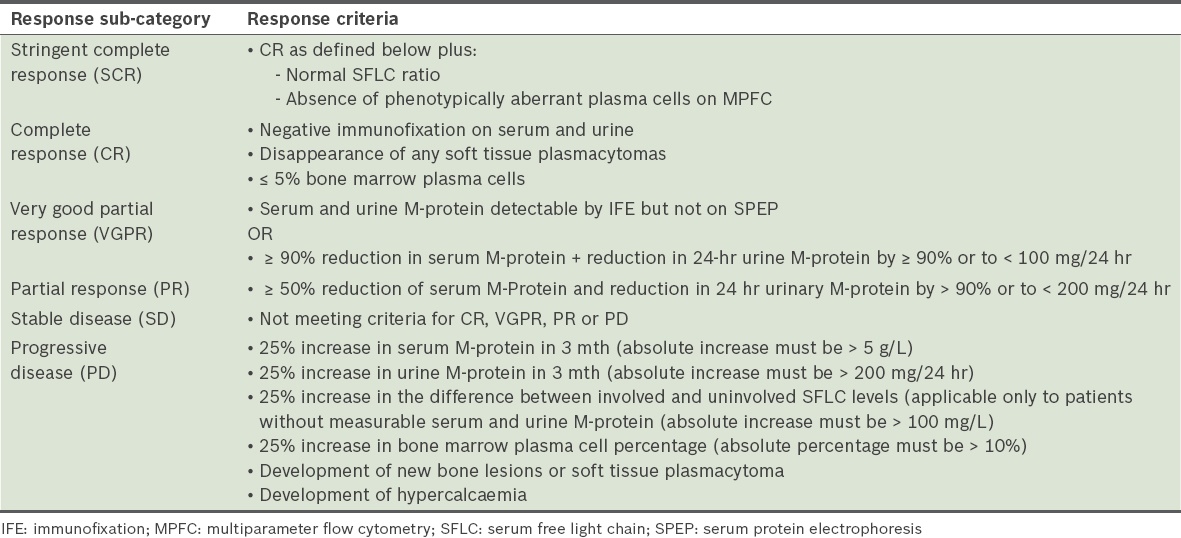
Response definitions
The response definitions are based on the uniform response criteria recommended by the IMWG, as summarised in
Table 6
International Myeloma Working Group definitions of response categories.
Choice of induction therapy
Cavo et al demonstrated that bortezomib, thalidomide and dexamethasone (VTD) produced a significantly higher rate of complete remission before stem cell transplantation (SCT) compared to thalidomide and dexamethasone.(46) Bortezomib, cyclophosphamide and dexamethasone (VCD) has also been shown to produce impressive response rates and survival.(47,48) A meta-analysis of Phase 3 trials comparing bortezomib and non-bortezomib-containing induction regimens showed a superior response rate and progression-free survival (PFS) for the bortezomib-containing regimens.(49) Therefore, we recommend that PI-based induction should be considered in all patients.
There is growing evidence that VTD may be superior to VCD. This was shown in a retrospective analysis by Leiba et al(50) and, more recently, in a prospective randomised study by the Intergroupe Francophone du Myelome (IFM).(51) At present, we would recommend both VTD and VCD as options for induction therapy, depending on the physician’s choice. If PI-based induction therapy is not possible, at least one novel agent should be a component of the induction regimen.(40) The bortezomib, lenalidomide and dexamethasone (VRD) regimen has shown activity in high-risk patients,(52,53) but has not been shown to be superior to VCD or VTD. VRD may be considered in certain high-risk patients, although the significant cost of this protocol should also be considered.
We suggest the following protocols as options for PI-based induction therapy:
-
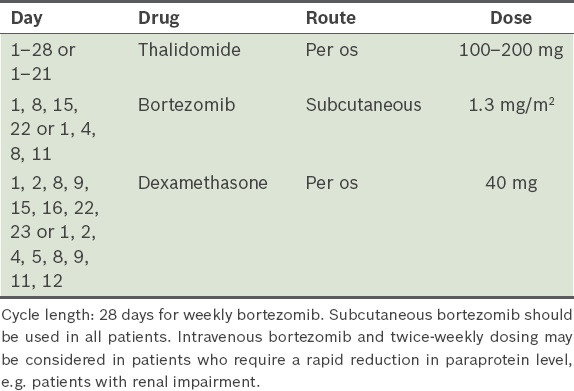
Bortezomib, thalidomide and dexamethasone, i.e. VTD (
Table 7)(46) Table 7
VTD (bortezomib, thalidomide and dexamethasone) protocol.
-
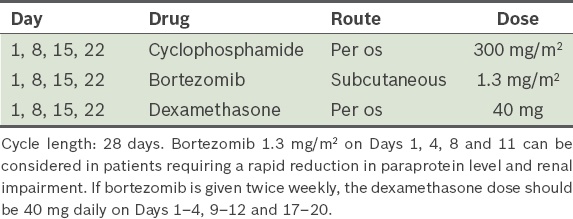
Bortezomib, cyclophosphamide and dexamethasone, i.e. VCD (
Table 8, modified from Reeder et al(47)) Table 8
VCD (bortezomib, cyclophosphamide and dexamethasone) protocol.
-
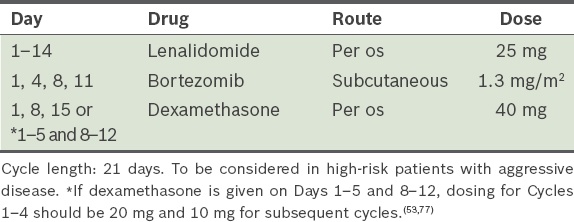
Bortezomib, lenalidomide and dexamethasone, i.e. VRD (
Table 9)(52,53) Table 9
VRD (bortezomib, lenalidomide and dexamethasone) protocol.
Options for patients for whom proteasome inhibitor-based induction therapy is not possible
The intensive therapy arm of the UK Medical Research Council (MRC) IX study showed that cyclophosphamide, thalidomide and dexamethasone (CTD) was superior to cyclophosphamide, vincristine, doxorubicin and dexamethasone in terms of response rate.(54) For patients who, for whatever reason, are not able to receive PI-based induction, we recommend that at least one novel agent be included in the induction regimen.
We recommend the following treatment protocols as options when PI-based induction is not possible:
-
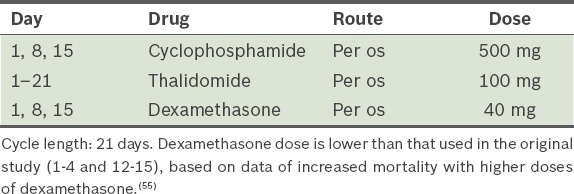
Cyclophosphamide, thalidomide and dexamethasone, i.e. CTD (
Table 10).(54) (Dexamethasone dose is lower than that used in the original study [Days 1–4 and 12–15], based on data of increased mortality with higher doses of dexamethasone.)(55) Table 10
CTD (cyclophosphamide, thalidomide and dexamethasone) protocol.
-
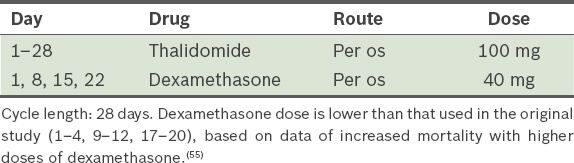
Thalidomide and dexamethasone, i.e. TD (
Table 11).(56) (Dexamethasone dose is lower than that used in the original study [Days 1–4, 9–12 and 17–20], based on data of increased mortality with higher doses of dexamethasone.)(55) Table 11
TD (thalidomide and dexamethasone) protocol.
Number of cycles of induction and response before stem cell harvest
The depth of response pre-transplant correlates with event-free survival and overall survival, with patients who show a CR having the best outcome.(57,58) The minimum response required before proceeding to ASCT is a partial response (PR). If a PR is not achieved after four cycles of induction therapy, a further two cycles should be considered. If a PR is not achieved after six cycles or there is progressive disease at any time point during induction, a change of therapy is recommended.(12,40)
Mobilisation chemotherapy and stem cell collection
High-dose cyclophosphamide (Cy) at 4–7 g/m2 with granulocyte colony-stimulating factor (GCSF) has been shown to be effective for haematopoietic progenitor cell mobilisation despite associated haematologic toxicity.(59) Vinorelbine 25 mg/m2 in combination with Cy 1,500 mg/m2 (Vino-Cy) was shown to be comparable to Cy mobilisation in a study using historical controls.(12,60) We recommend one of the following mobilisation protocols: (a) vinorelbine 25 mg/m2 on Day 1, Cy 1,500 mg/m2 on Day 2 and pegylated GCSF 6 mg on Day 4; or (b) Cy 1,500 mg/m2 on Days 1 and 2 and GCSF 10 mcg/kg/day from Day 5 onwards.
A haematopoietic progenitor cell collection adequate for two SCTs should be the target; this is conventionally accepted to be greater than 5 × 106 per kg/body weight.(61)
Conditioning regimen
ASCT is the standard of care for transplant-eligible patients with MM. This was demonstrated in two pivotal randomised studies in the pre-novel agent era, as well as two studies in the novel agent era.(41-43,62) Melphalan 200 mg/m2 is the standard conditioning regimen.(41) The addition of bortezomib to melphalan conditioning was shown in a Phase II study to be associated with higher CR rates compared to historical controls.(63) However, there is no randomised trial data to show the benefit of this combination, and thus we do not recommend it outside of clinical trials.
Single versus double autologous stem cell transplantation
A randomised study by the IFM showed that tandem ASCT results in overall survival benefit for patients who achieved less than a VGPR after their first ASCT.(64) This study was, however, in the pre-novel agent era, and there is limited data to support this practice in the novel agent era. Tandem ASCT should not be routinely offered, but instead considered only in patients who have achieved less than a VGPR after their first ASCT, especially in patients with high-risk disease.
Disease monitoring
We recommend that the following parameters be monitored after each cycle of treatment:(12) M-protein level; FBC; renal function; calcium; immunoglobulin level (if immunoglobulin A MM); SFLC level (instead of M-protein quantification and immunoglobulin level) for light chain myeloma; and bone marrow studies if aiming to confirm CR or investigate unexplained cytopenia.
Role of consolidation therapy
As there is no clear evidence of an overall survival benefit from bortezomib or lenalidomide consolidation, they are not routinely recommended. Bortezomib and lenalidomide have both been shown to prolong PFS and are therefore options for consolidation in selected patients; in those with only a PR after ASCT, two further cycles of a regimen similar to the induction can be given for consolidation.(65,66)
Role of maintenance therapy
Lenalidomide maintenance has been shown to prolong PFS and overall survival after ASCT in one randomised clinical trial.(67) It should, therefore, be considered an option for patients after a thorough discussion of the risks, benefits and costs. In view of the risk of second primary malignancy, the duration of lenalidomide maintenance should be limited to two years. Thalidomide maintenance has also been shown to prolong PFS after ASCT in patients who achieved less than a VGPR.(68) Thus, it may be considered in patients who have achieved less than a VGPR after ASCT. The duration of thalidomide maintenance should be limited to one year in view of the risk of neuropathy.(69)
Single-agent bortezomib maintenance has only been assessed in one prospective Phase III study where the standard arm was treated with vincristine, adriamycin, dexamethasone induction, high-dose therapy and thalidomide maintenance. It is, therefore, not possible to recommend bortezomib maintenance outside of a clinical trial at this point.(42)
Role of allogeneic transplant
Allogeneic SCT is currently the only curative therapy available for MM; however, it is associated with high transplant-related mortality of 20%–30%.(70) Allogeneic SCT may be considered in an upfront setting or at first relapse for young, fit patients with high-risk disease, especially 17p del. Patients who are very young (e.g. < 40 years) with standard-risk disease are another group for whom this approach is a consideration.(71)
Conclusion
ASCT remains the standard of care for transplant-eligible MM patients. The depth of response pre-ASCT correlates with long-term outcome.(57) Delivering the optimal novel agent-based induction regimen (bearing in mind the cost of these drugs) can be a challenge, as options for funding vary between institutions. We recommend that patients be included in high-quality randomised studies if they are eligible. With improved risk stratification and the availability of highly potent novel agents, the role of ASCT in MM is likely to evolve over the next few years.
IV. MANAGEMENT OF TRANSPLANT-INELIGIBLE PATIENTS
Patient selection
Patients who are > 65 years of age or have significant comorbidities are generally ineligible for high-dose therapy. Categorisation of their fitness to receive intensive treatment should be based on comorbidities and performance status, which may be assessed using a validated score (
Table 12
Fitness for therapy patient categorisation.

Frontline management strategy for newly diagnosed transplant-ineligible patients
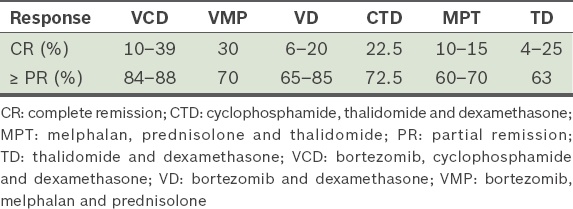
The goal of therapy is to maximise treatment responses while minimising treatment-related toxicities. All patients should be considered for enrolment into clinical trials when they are available. The inclusion of a novel agent in frontline therapy for transplant-ineligible MM patients has been shown to result in more rapid disease control as well as improved survival. These regimens are also generally well tolerated.(74) Outside of a clinical trial, we recommend PI-based induction, given its superior response rates and overall survival data.(75) The FIRST trial showed that lenalidomide and dexamethasone (RD) is superior to melphalan, prednisolone and thalidomide (MPT) as a frontline regimen for transplant-ineligible MM patients. It is noteworthy that this effect was more apparent for continuous therapy with RD and that there has been no randomised study comparing RD to a PI-based regimen.(76) However, RD should be considered a potential frontline option for transplant-ineligible MM patients. The VRD regimen was compared against RD for transplant-ineligible MM patients in a randomised study, which showed the superiority of VRD.(77) Although this represents another potential treatment option, VRD has not been compared to other bortezomib-based combinations. Suggested treatment protocols are summarised and the regimens described in
Table 13
Frontline treatment options for transplant-ineligible multiple myeloma patients.
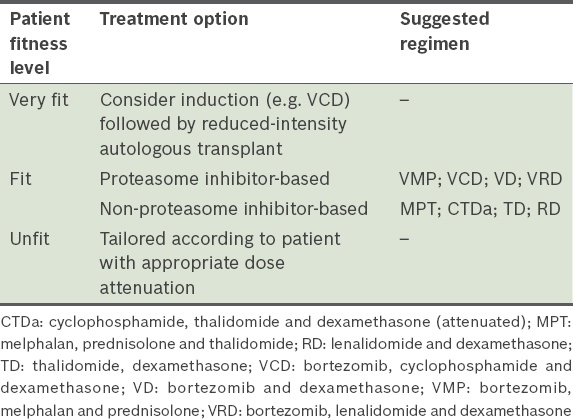
Table 14
Comparison of response rates with the protocols described.
Monitoring of response to therapy
We recommend that M-protein quantification, immunoglobulin level, FBC, renal function and calcium levels should be monitored after each cycle. SFLC levels, instead of M-protein quantification, may be used for light chain myeloma. Once an M-protein plateau is reached, M-protein quantification may be performed every 2–3 months. Bone marrow studies should be used to confirm complete remission when applicable or to investigate unexplained cytopenia.(12)
Duration of induction therapy
We recommend treatment until at least a PR and m-band plateau is achieved. This is defined as three consecutive m-band results, which qualify for at least a PR, and being stable with no new CRAB features. As a guide, 9–12 cycles of therapy is recommended if no maintenance is planned and 6–9 cycles if maintenance is planned. The decision to continue treatment should be balanced against toxicity.(78)
Maintenance therapy
Thalidomide and lenalidomide maintenance have been shown to prolong progression-free survival, but the overall survival benefit remains controversial.(69,79) Thalidomide maintenance may be considered, but it should be limited to a one-year duration in view of the risk of neuropathy.(69) The FIRST trial has demonstrated the efficacy and tolerability of continuous RD in transplant-ineligible patients. In this study, the continuous RD arm showed improved response rates, progression-free survival and overall survival at interim analysis as compared to MPT.(76) Single-agent bortezomib maintenance has not been assessed in the transplant-ineligible population. Bortezomib and thalidomide (VT) or bortezomib and prednisolone (VP) maintenance following bortezomib, thalidomide and prednisolone (VTP) or bortezomib, melphalan and prednisolone (VMP) induction showed better response and progression-free survival as compared to the VMP regimen without maintenance, but there was no overall survival benefit. However, comparison of VP and VT in this study is difficult in view of the different induction regimens used.(80) It is, therefore, not possible to recommend bortezomib maintenance at this point.
The achievement of an overall survival benefit through maintenance therapy may be difficult to demonstrate due to the availability of effective salvage treatment at relapse.(81) There is emerging evidence to suggest the efficacy of the continuous use of RD rather than a fixed duration of an alkylator-based regime.(76) As for other induction regimes, there is currently insufficient data to justify the routine use of maintenance therapy outside of clinical trials. A thorough discussion of the financial aspects and quality-of-life considerations should be undertaken with the patient when offering the option of maintenance therapy.
Suggested protocols
We suggest the following treatment protocols as options for transplant-ineligible patients with MM. The protocols are described in detail in the respective tables.
-
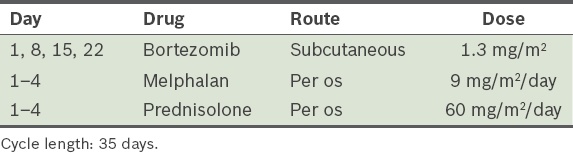
Bortezomib, melphalan and prednisolone (VMP)(75,80) (
Table 15) Table 15
VMP (bortezomib, melphalan and prednisolone) protocol.
-
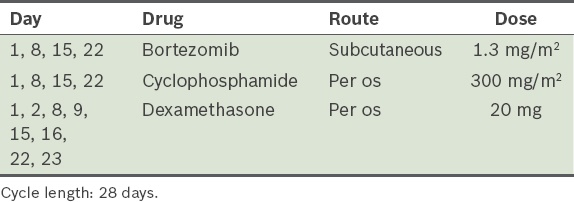
Bortezomib, cyclophosphamide and dexamethasone (VCD)(47,48) (
Table 16) Table 16
VCD (bortezomib, cyclophosphamide and dexamethasone) protocol, as modified from Reeder et al.(47)
-
Bortezomib and dexamethasone (VD)(82) (
Table 17) -
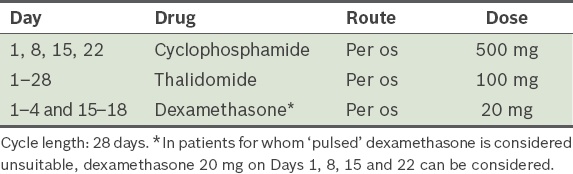
Cyclophosphamide, thalidomide and dexamethasone attenuated (CTDa)(54) (
Table 18) Table 18
CTDa (cyclophosphamide, thalidomide and dexamethasone) attenuated protocol, as modified from Morgan et al.(54)
-
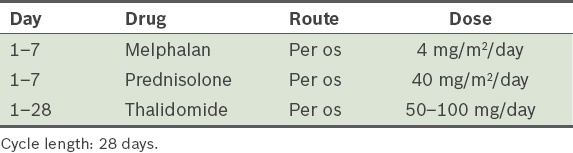
Melphalan, prednisolone and thalidomide (MPT)(83) (
Table 19) Table 19
MPT (melphalan, prednisolone and thalidomide) protocol, as modified from Palumbo et al.(83)
-
Thalidomide and dexamethasone (TD)(84) (
Table 20) Table 20
TD (thalidomide and dexamethasone) protocol.
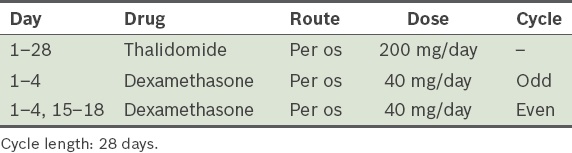
-
Bortezomib, lenalidomide and dexamethasone (VRD) (
Table 9)(77) -
Lenalidomide and dexamethasone (RD)(76) (
Table 21) Table 21
RD (lenalidomide and dexamethasone) protocol.

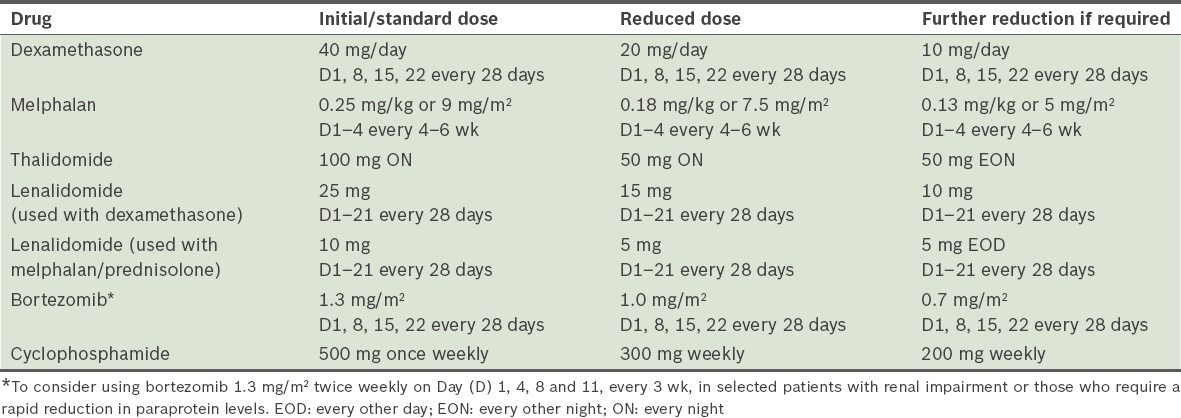
Dose adjustment guidelines, depending on patient fitness, are summarised in
Table 22
Dose adjustments in elderly patients with multiple myeloma, as adapted from Palumbo et al.(85)
Conclusion
The management of transplant-ineligible MM patients should be individualised. The approach should take into consideration the patient’s disease burden, comorbidities and the likelihood of treatment-related toxicities. While one of the goals is to attain the best disease response with treatment, the treating physician should not neglect the goals of minimising adverse effects and providing adequate supportive care.
The introduction of novel agents – bortezomib, thalidomide and lenalidomide – has changed the treatment practices for transplant-ineligible MM. These treatment regimens are effective, with manageable toxicities. The armamentarium has since undergone exponential expansion with the advent of second-generation PIs (e.g. ixazomib and carfilzomib) and immunomodulators (e.g. pomalidomide), as well as the introduction of immunotherapy (e.g. daratumumab and checkpoint inhibitors).(86-90) However, while such potent drugs may be available, the physician is tasked to justify the added benefits of administering these treatments by weighing them against the financial burdens they may bring.
V. DRUG TOXICITY AND DOSE ADJUSTMENTS
This section provides guidance on the dosages and administration of drugs commonly used in treating MM. Recommendations for dose adjustments in renal and hepatic impairment, as well as information on common toxicities, are also provided. The source of this information is the manufacturers’ package insert and recent publications in the field. We recommend that an oncology-trained pharmacist be responsible for dispensing these agents.
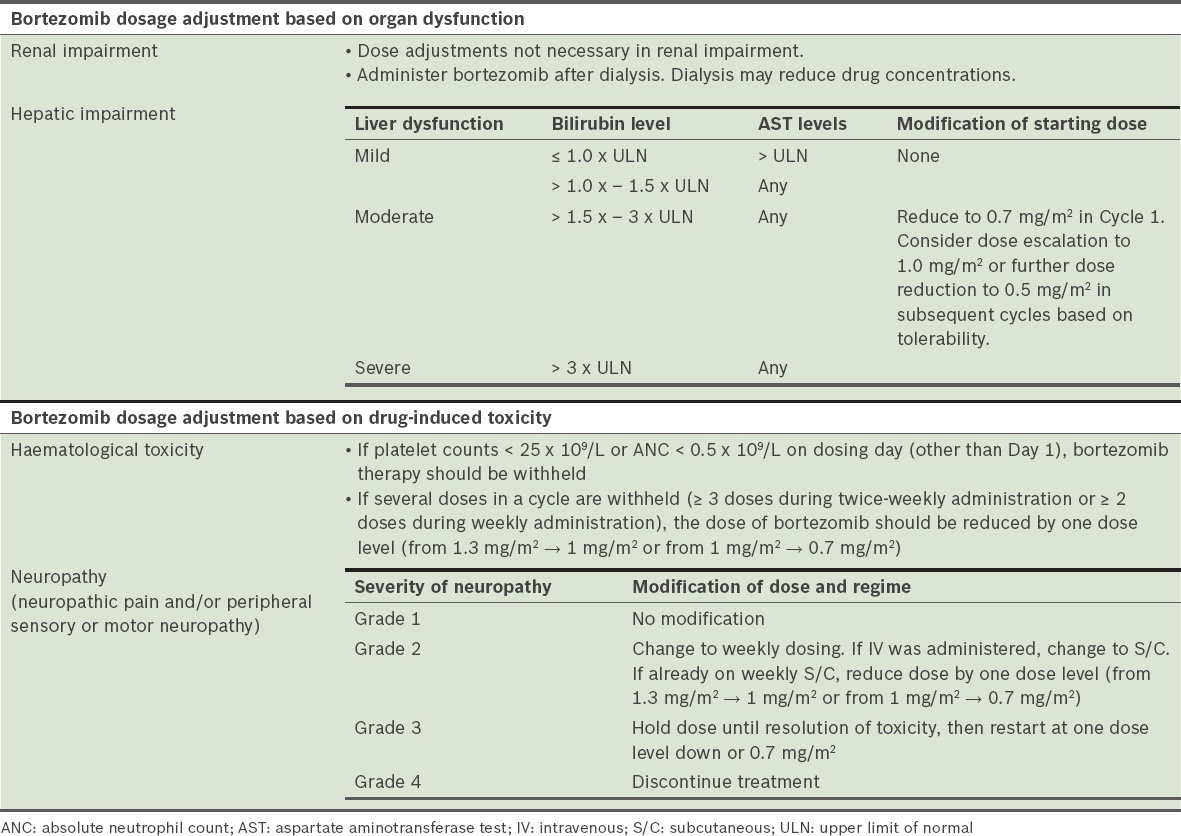
Bortezomib
Bortezomib is currently the first choice for standard PI-based induction. For Cycle 1, FBC should be assessed 24 hours prior to the first and third dose of bortezomib. For subsequent cycles, FBC should be assessed at least twice per cycle. More regular monitoring may be considered on a case-by-case basis, depending on the severity of cytopenia at the beginning of the cycle and concurrent cytotoxic therapy. Prior to initiating a new cycle of therapy (
Table 23
Dose adjustment and toxicity of bortezomib (VELCADE® [bortezomib] injection package insert).
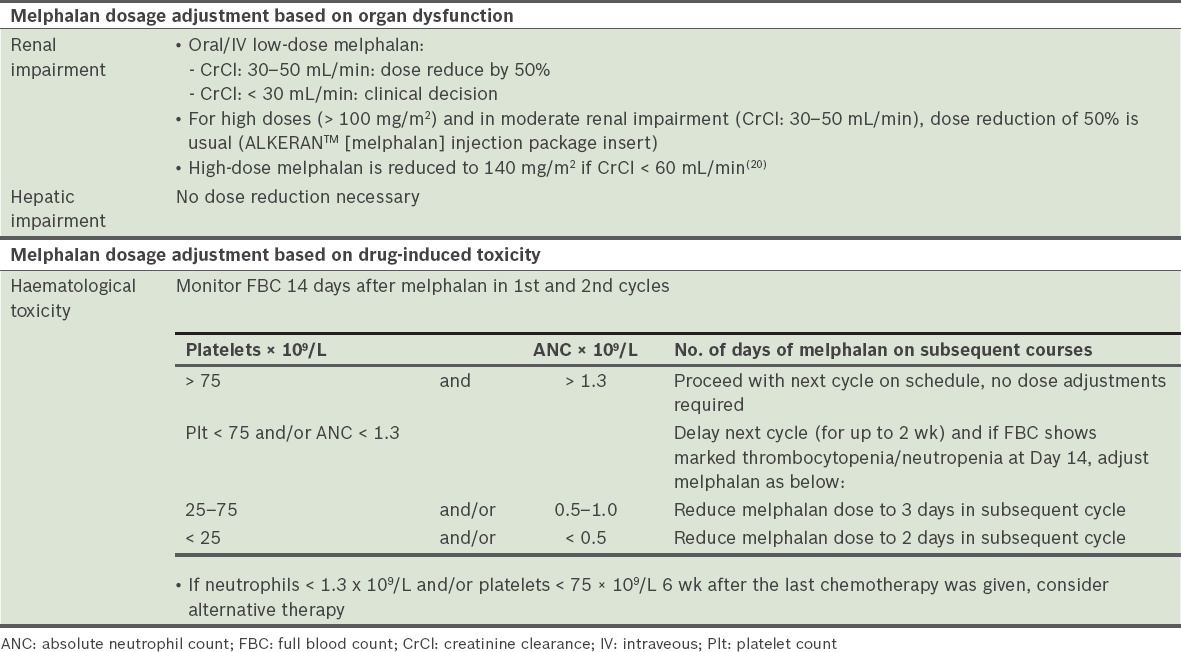
Melphalan
Melphalan is indicated as part of the VMP and MPT protocols, as well as in conditioning for SCT. Significant haematologic and gastrointestinal toxicities are well known. Dose adjustment for renal impairment is recommended (
Table 24
Dose adjustment and toxicity of melphalan [ALKERAN™ (melphalan) injection package insert].
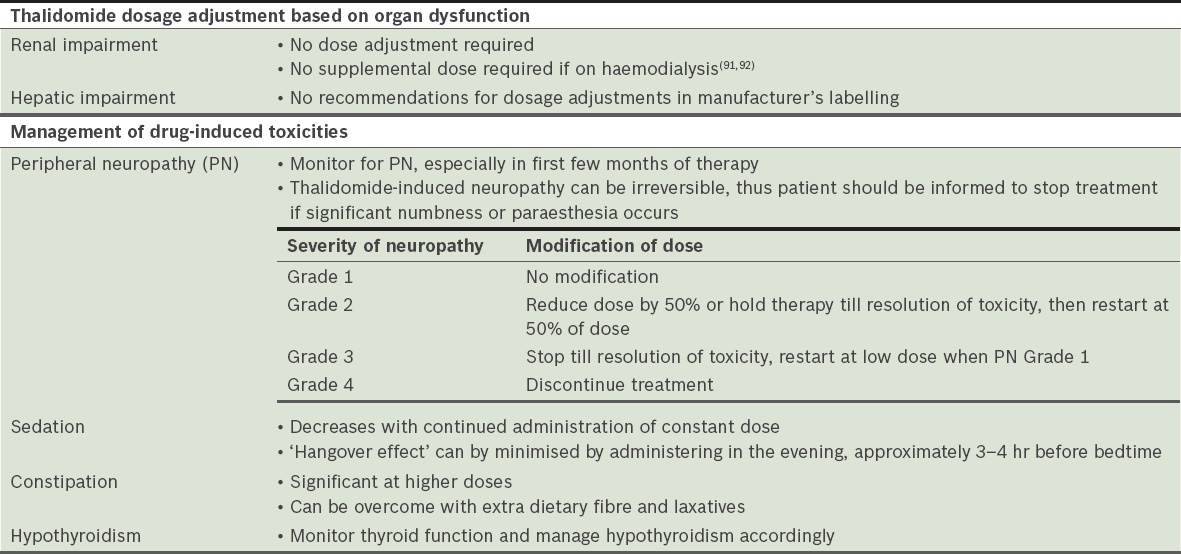
Thalidomide
Thalidomide is an important component of multiple treatment protocols in both transplant-eligible and -ineligible patients. Peripheral neuropathy, constipation, sedation and hypothyroidism are significant toxicities (
Table 25
Dose adjustment and toxicity of thalidomide.
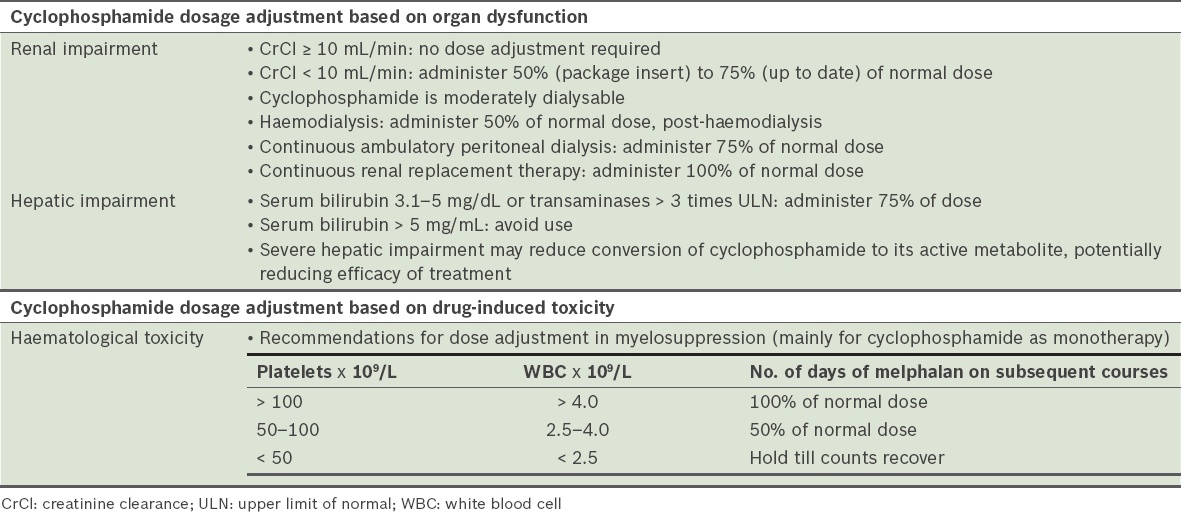
Cyclophosphamide
Cyclophosphamide is indicated in the VCD and CTD regimens. The main toxicity is haematologic, and adjustment is required for renal and hepatic impairment (
Table 26
Dose adjustment and toxicity of cyclophosphamide (ENDOXAN® [cyclophosphamide] package insert).
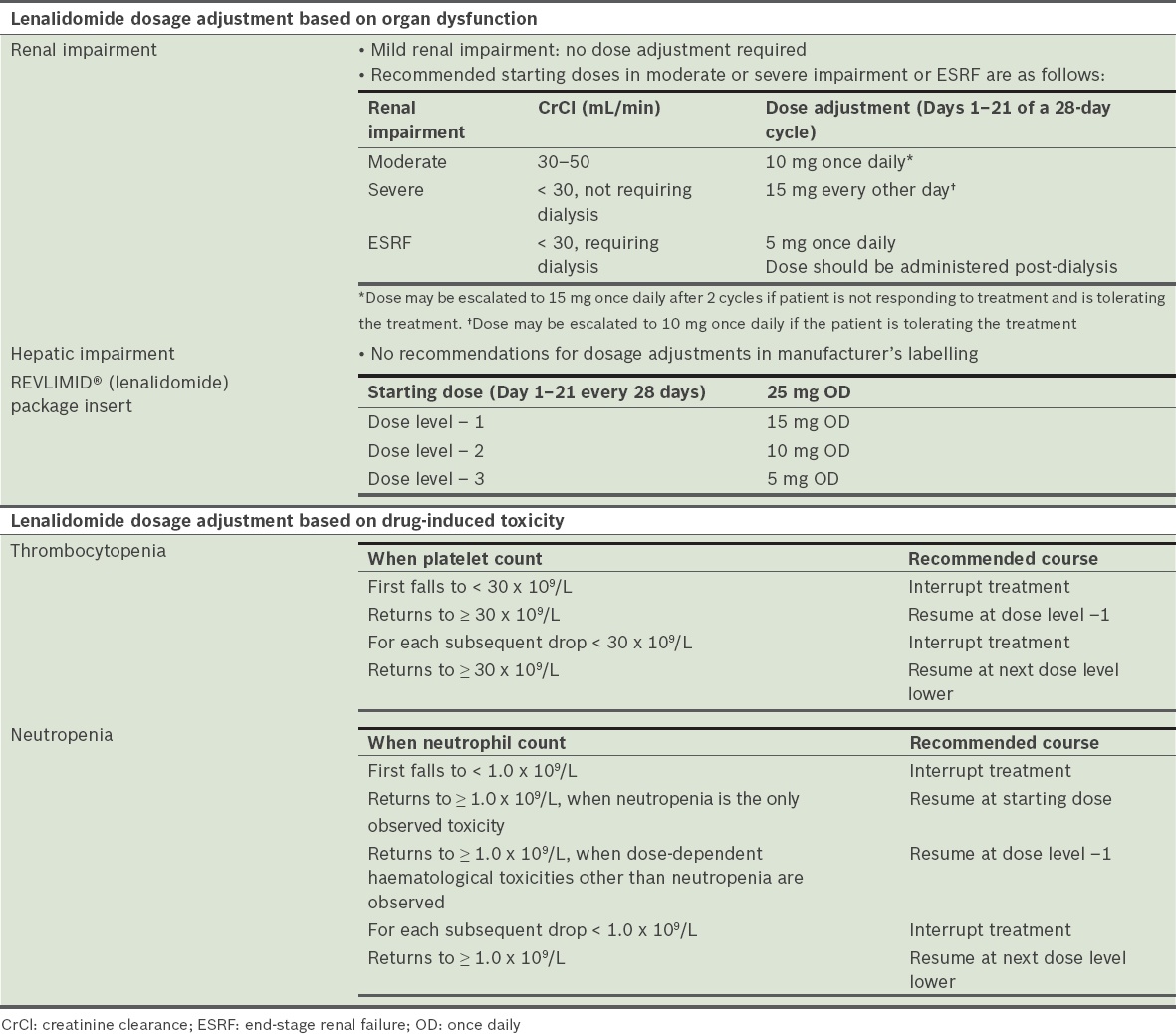
Lenalidomide
The recommended starting dose of lenalidomide is 25 mg once daily on Days 1–21 of a 28-day cycle. The recommended dose of dexamethasone is 40 mg orally once daily on Days 1, 8, 15 and 22.(55) Prior to initiating a new cycle of therapy (
Table 27
Dose adjustment and toxicity of lenalidomide.
Carfilzomib
Carfilzomib (exemption supply, i.e. check with institutional pharmacy for cost and forms for application of drug) is administered as an intravenous injection over 2–10 minutes, on two consecutive days each week for three weeks (Days 1, 2, 8, 9, 15 and 16), followed by a 12-day rest period every 28 days. In Cycle 1, carfilzomib is given at a dose of 20 mg/m2. If tolerated, the dose should be escalated to 27 mg/m2 in subsequent cycles. Prior to each dose in Cycle 1, administer 250–500 mL of intravenous normal saline and an additional 250–500 mL of intravenous fluids as needed, following carfilzomib administration. Continued intravenous hydration is required in subsequent cycles to ensure adequate hydration while preventing fluid overload.
Premedication with oral or intravenous dexamethasone 4 mg prior to all doses of carfilzomib in Cycle 1, and prior to all doses during the first cycle of dose escalation to 27 mg/m2, is required to reduce the incidence and severity of infusion-related reactions. Dexamethasone premedication should be reinstated if these symptoms develop or reappear during subsequent cycles (
Table 28
Dose adjustment and toxicity of carfilzomib.

CONCLUSION
The details regarding dose adjustments for each drug are described in the aforementioned tables. We suggest that prescribing physicians refer to the relevant drug package insert for a more comprehensive review of toxicity and dose adjustments. We reiterate the importance of good communication and collaboration with the haemato-oncology pharmacy team when using these agents.