Abstract
We present the 2021 Singapore Paediatric Resuscitation Guidelines. The International Liaison Committee on Resuscitation’s Pediatric Taskforce Consensus Statements on Science and Treatment Recommendations, which was published in October 2020, and the updated resuscitation guidelines from the American Heart Association and European Resuscitation Council, were reviewed and discussed by the committee. These recommendations were derived after deliberation of peer-reviewed evidence updates on paediatric resuscitation and took into consideration the local setting and clinical practice.
LOCAL EPIDEMIOLOGY
Based on the paediatric sub-study of the Pan-Asian resuscitation outcomes study (i.e. PAROS), local paediatric out-of-hospital cardiac arrest (OHCA) from 2009 to 2012 occurred in a young population (median age two years; interquartile range 0–17 years).(1) Bystander cardiopulmonary resuscitation (CPR) rate was 35.6%, and most CPRs were rendered by family members. 20.3% of the paediatric patients went to an emergency department (ED) in their own transport. Survival-to-discharge rate was 3.4%, with only 1.7% of paediatric patients having favourable neurological outcomes (Cerebral Performance Category score 1–2). While things would have likely improved since 2012, it is important to continue to advocate for community efforts to ensure optimal care and better outcomes for the paediatric population in the pre-hospital setting. For paediatric in-hospital cardiac arrest (IHCA), the local survival outcomes from 2009 to 2014 have been reported to be much higher, at 45.6% survival-to-hospital discharge.(2)
AGE GROUP CONSIDERATIONS
A neonate is defined as a patient aged ≤ 28 days, while an infant is defined as a patient aged below one year. A child is any patient aged 1–12 years, and an adolescent is a patient aged above 12 years. Unless otherwise stated, ‘paediatric’ in our recommendations includes any patient aged below 18 years, and excludes newborns and neonates that are managed in neonatal units.
Neonates (age ≤ 28 days)
All newly born infants (i.e. newborns) will be resuscitated using the Singapore newborn life support algorithm. All neonates managed in neonatal units, regardless of postnatal age, will also be resuscitated following the Singapore newborn life support algorithm.
The resuscitation science is in equipoise on the comparative use of newborn and paediatric life support algorithms for improved long-term survival and neurological outcomes in infants ≤ 28 days of age, who have fully transited from the fetal circulation after delivery and have been discharged to the community from neonatal units or nurseries. We took into consideration established pre-hospital, ED and hospital workflows in our recommendations.
We suggest that it is reasonable for neonates (age ≤ 28 days), excluding newborns, in the community, ED or paediatric units, to follow the paediatric basic and advanced life support algorithms.
Adolescents
Basic life support for community first responders
Previously, in the 2015 paediatric basic life support guidelines, the American Heart Association (AHA) and European Resuscitation Council (ERC) suggested the use of the adult basic life support sequence for adolescents with signs of puberty, which would be more likely if they are aged > 12 years, as compared to > 8 years.(3,4) However, in the most recent resuscitation guidelines,(5,6) the age group has been revised up to 18 years of age. In view of this shift, we have extended our current paediatric basic life support to ≤ 12 years of age from the previous ≤ 8 years. A local radiological study conducted in the paediatric population has reported that a simulated chest compression of 6 cm (i.e. upper limit of adult chest compression guidelines) would result in a potential for over-compression in 5% of children aged > 8 years as compared to 2% of children aged > 12 years.(7) While there is no direct evidence to suggest that adolescents would benefit more from the paediatric basic life support sequence and that laypersons may be more familiar with the adult basic life support algorithm, we have included ventilation as part of the basic life support for adolescents aged > 12 years to provide guidance to healthcare providers and community first responders who are able and willing to provide ventilation.
For healthcare providers and community first responders who are able and willing, we advocate that ventilation should be provided as part of CPR, even if the casualty is > 12 years of age.
Advanced life support
For advanced life support, the resuscitation science in adolescents is in equipoise on the comparative use of adult versus paediatric advanced life support algorithms for improved long-term survival and neurological outcomes, especially for older adolescents in the 16–18 years age group. We took into consideration our locally established pre-hospital, ED and hospital workflows in our recommendations for advanced life support in paediatrics.
It is reasonable for patients aged ≤ 18 years in our local paediatric EDs and units to be resuscitated following paediatric advanced life support algorithms. It is also reasonable that patients aged ≥ 16 years in our local general EDs and non-paediatric units be resuscitated with adult advanced life support algorithms.
PAEDIATRIC CHAIN OF ‘NEUROLOGICALLY INTACT’ SURVIVAL
Increasingly, guidelines have been targeted at not just survival outcomes but also long-term neurological outcomes for paediatric cardiac arrest victims.(8,9) We have modified the term ‘paediatric chain of survival’ to ‘paediatric chain of neurologically intact survival’ to emphasise this. While the components are largely similar, we wanted to emphasise the strategies to optimise neurologically intact survival for paediatric cardiac arrest victims (
Fig. 1
Diagram shows the paediatric chain of neurologically intact survival.
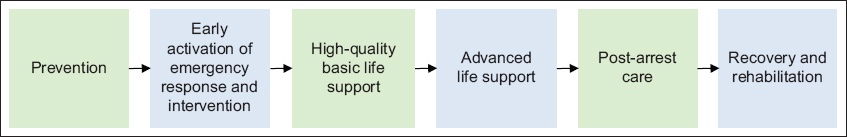
1. PREVENTION
Since 2010, there has been emphasis on preventive strategies and early recognition of pre-cardiac arrest states, as they may have greater clinical impact owing to poorer outcomes once paediatric cardiac arrests have occurred, especially for OHCA.(3-5,9) Injury prevention and preventive medical strategies such as vaccination and equitable access to healthcare services may be the most cost-effective measure to prevent morbidity and mortality in the paediatric population.
2. EARLY ACTIVATION OF EMERGENCY RESPONSE AND EARLY INTERVENTION
Prompt activation of emergency response and intervention by earlier identification of critically ill or injured paediatric patients are essential to improve patient outcomes.
Out-of-hospital recognition of cardiac arrest
Laypeople should call the ambulance service (‘995’), which will direct them to a dispatch officer who can aid them in the evaluation of a paediatric patient in cardiac arrest. Timely activation of ambulance personnel to provide on-site evaluation and management is crucial. It is important that this simplified dispatch-assisted process is similar to the initial steps of evaluating an adult with suspected cardiac arrest.(10)
In-hospital identification of high-risk groups
For institutional settings, paediatric early warning scores (PEWS) have been used to identify high-risk paediatric patients who may require closer monitoring or early intervention. Activation of medical emergency teams (METs) or rapid response teams (RRTs) that follow up with these high-risk patients identified by PEWS has been met with varying success.(11-13) It may be important to validate these scores in the local setting and to modify them according to local risk factors, which may be setting-dependent. We suggest the use of PEWS or similar systems to identify high-risk paediatric patients, with timely review by a paediatric MET/RRT or similar systems in hospitals with inpatient paediatric units.
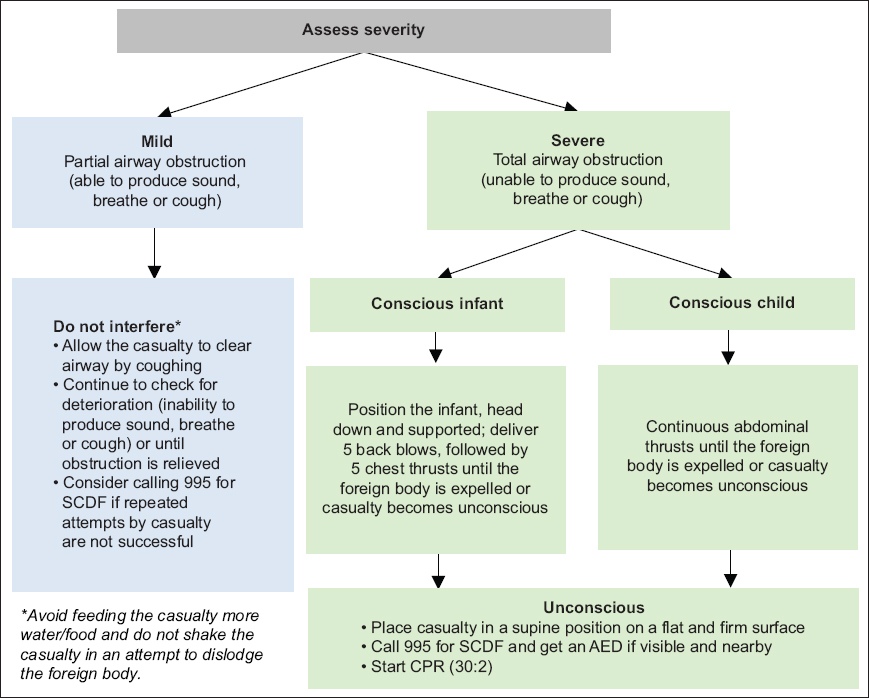
Management of foreign body airway obstruction in infants and children
Foreign body aspiration in paediatrics, if detected and managed early, can prevent significant morbidity and mortality. While we aim to synchronise the paediatric and adult algorithms for foreign body aspiration, we strongly recommend the provision of ventilation in the paediatric population for unconscious victims. For rescuers who are willing and able, opening the airway to remove the foreign bodies and provide ventilation should be performed in unconscious infants and children with foreign body airway obstruction (FBAO). The algorithm for FBAO for infants and children is shown in
Fig. 2
Flowchart shows the algorithm for foreign body airway obstruction. AED: automated external defibrillator; CPR: cardiopulmonary resuscitation; SCDF: Singapore Civil Defence Force
3. HIGH-QUALITY BASIC PAEDIATRIC LIFE SUPPORT
Paediatric basic life support should be used in the following age groups: excluding newborns, any infant who was previously discharged from neonatal units; any child aged ≤ 12 years; and adolescents > 12 years (rescuers who are able and willing should provide ventilations if they feel that the adolescent casualty is child-sized or may benefit from the provision of ventilations, e.g. drowning, asthma).
Components of high-quality paediatric basic life support
The five main components of high-quality chest compressions are: adequate chest compression depth; optimal chest compression rate; minimising interruptions in CPR (minimise proportion of hands-off time to chest compressions during paediatric cardiac arrest); allowing full chest recoil between compressions; and provision of ventilation but avoiding hyperventilation (age-specific). The algorithm for paediatric basic life support is shown in
Fig. 3
Flowchart shows the algorithm for Paediatric Basic Life Support CPR + AED 2021. AED: automated external defibrillator; CPR: cardiopulmonary resuscitation; SCDF: Singapore Civil Defence Force
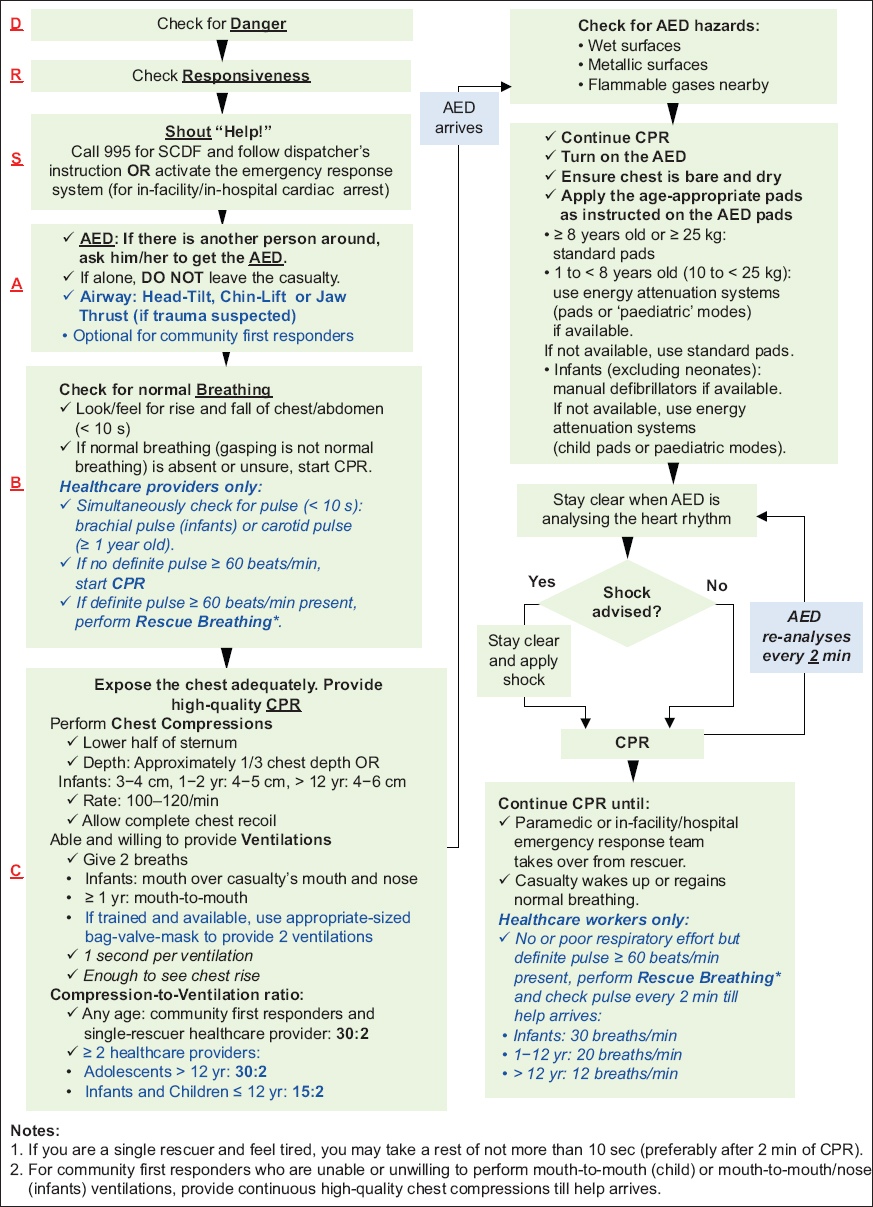
Box 1
Recommended sequence of action in Paediatric Basic Life Support.

Common initial management of cardiac arrest: activation of emergency medical services
To encourage bystander CPR (i.e. community first responders), we advocate a common pathway initial algorithm for both adult and paediatric patients to ensure familiarity. The need for early activation of the emergency medical services (EMS) system (‘995’) or code teams is emphasised. Dispatch assistance has been successfully demonstrated to assist in identification of cardiac arrest states and administration of CPR, with resultant improved clinical outcomes.(10,14-16)
The Singapore Civil Defence Force dispatch service also activates nearby volunteer community first responders to obtain the nearest automated external defibrillator (AED) and mobilises them to the casualty’s location. Thus, there is no need for lone rescuers to leave the paediatric cardiac arrest victim’s side to obtain AEDs. Community responders should focus on providing ongoing CPR with assistance from the dispatch service through mobile phones or other mobile communication devices. In the rare occasion that mobile phones are not available and there is only a single rescuer with no one else around to help, the single rescuer should attempt two minutes of CPR first, then seek nearby assistance or obtain phone access and return to the casualty immediately to continue CPR.
Opening airway and ventilation
Airway opening is essential in the evaluation of breathing, as this is used as a surrogate of having ‘signs of life’. Unconscious infants and younger children who are not in cardiac arrest may have airway obstruction owing to their relatively large head and proportionally larger tongue (especially in infants).(17) Opening of the airway is emphasised in paediatrics, as it may restore normal breathing and can be life-saving in itself. However, this may be challenging for community responders and, while optional, airway opening is highly encouraged.
Recognition of cardiac arrest
Recognition of paediatric cardiac arrest in the community – even with dispatch assistance – is challenging.(18) A local study found improved accuracy when the first responder’s hand is placed on the abdomen of unconscious adult patients to identify cardiac arrest with assistance from the dispatch ambulance service.(19) This method has been used in the paediatric population, especially in infants and younger children, to count respiration, as these casualties tend to be abdominal breathers. We suggest that this should be also endorsed in the paediatric population as part of evaluation for breathing.
Accuracy of pulse check by healthcare workers
Pulse check is not reliable as a sign of a perfusing cardiac rhythm, even among healthcare workers.(20-23) Therefore, as with the 2016 Singapore paediatric resuscitation guidelines, pulse check is limited to healthcare providers and provides information only if clearly detected within ten seconds. This is done simultaneously when assessing for breathing. Alternative methods for pulse check that healthcare providers can employ to manage a patient with cardiac arrest include cardiac auscultation and checking of the femoral pulse.(24-26) Use of bedside ultrasonography to evaluate for cardiac standstill can be considered by trained healthcare providers, but evidence for its use in paediatric patients is extremely limited and remains investigational.(27)
Chest compression technique
Healthcare providers are encouraged to use the encircling method, as it provides better compressions.(28) However, the two-thumb encircling method has been associated with increased incidence of incomplete recoil. Whichever method (two-finger technique or two-thumb encircling technique) is used, adequate depth and full recoil should be ensured.
Chest compression depth
A local paediatric radiological study that looked at chest dimensions supported the use of ‘approximately’ one-third chest depth and the local paediatric compression absolute depth targets (if compression depth can be measured using CPR feedback devices). Current AHA and ERC absolute chest compression depth recommendations (approximately 4 cm for infants and approximately 5 cm for children) may risk over-compression in our local population.(5) Two adult North American studies, by Duval et al(29) and Stiell et al,(30) observed that the optimal compression depth was less than that recommended by the AHA (i.e. 5–6 cm), with peak survival and favourable neurological outcomes at chest compression depths of 4.7 cm and 4.56 cm, respectively.(29,30) Therefore, we reaffirm our current paediatric chest compression recommendations for absolute depth targets, especially for infants and younger children – infants: 3–4 cm; children 1–12 years: 4–5 cm; and adolescents > 12 years: 4–6 cm.
Emphasis on ventilation as part of paediatric basic life support
There is continued emphasis on ventilation in paediatric cardiac arrest. The vast majority of paediatric cardiac arrests are not due to cardiac causes, and provision of ventilation may be necessary to minimise the risk and duration of hypoxic ischaemic encephalopathy during the cardiac arrest event. A 2019 systematic review and meta-analysis (five observational studies with 14,427 participants) found improved clinical outcomes in favour of conventional CPR (chest compressions with ventilations) compared with the use of chest compression-only CPR provided by bystanders on paediatric OHCA casualties.(31) Bystander CPR with ventilations versus CPR without ventilations was associated with improved 30-day survival (odds ratio 1.49; 95% confidence interval 1.27–1.74) and improved 30-day neurological status (odds ratio 1.63; 95% confidence interval 1.30–2.04). A systematic review conducted in 2017 reported similar findings for the paediatric cardiac arrest population, that chest compression-only CPR was associated with poorer neurological and survival outcomes when compared to CPR with ventilations.(32)
We suggest that if rescuers are willing and able, ventilations should be provided as part of CPR to paediatric cardiac arrest casualties. However, if the rescuer is unable or unwilling to perform ventilation on paediatric cardiac arrest casualties, it is recommended to at least provide high-quality uninterrupted chest compressions until help arrives. While there is an emphasis on ventilation in paediatric life support, it is important that hyperventilation is avoided.
Chest compressions and ventilation ratios
A 2017 meta-analysis found less favourable survival and neurological benefits when uninterrupted chest compression were administered by EMS personnel on adult cardiac arrest casualties, as compared to interrupted compressions followed by ventilations.(33) Ventilation with the chest being compressed at the same time (air would be expelled from the lungs during compression delivery) without an advanced airway in situ may not be effective and may result in gastric insufflation. Given the importance of ventilation in paediatric cardiac arrest, we suggest that compressions and ventilations should be delivered synchronously in the 30:2 or 15:2 ratio until advanced airway placement.
Emphasis on public access defibrillators
Paediatric OHCA registry studies reported that the use of AED in paediatric cardiac arrest was associated with improved outcomes despite the lower frequency of shockable rhythms in paediatric OHCA.(34-37) Therefore, we recommend that laypersons follow a common basic life support algorithm with regard to access and application of AEDs for paediatric cardiac arrest casualties. The paediatric subcommittee further advocates the use of AEDs (with attenuation systems, if appropriate) for paediatric OHCA.
Paediatric dose-attenuating systems can be used in children aged < 8 years or weighing < 25 kg to reduce the energy dose delivered by the AEDs. This may be done either via a paediatric-specific (attenuated) pad-cable system or an AED with a paediatric mode or switch to select for half of the adult energy dose. Most paediatric-specific attenuation pads deliver 50–75 J. While we recommend the use of the most appropriate energy dose in the paediatric population, the use of standard AED pads can be life-saving when no paediatric energy attenuation systems are available.
For infants aged one month and older, if manual defibrillators are not immediately available, AEDs with dose attenuation systems (child pads or paediatric modes) are advised. If AEDs with energy attenuating systems are not available, consider the use of standard AEDs (comparative harm of not defibrillating shockable rhythms). There have been reported cases of safe and effective use of AEDs in infants and young children at an energy dose exceeding 4 J/kg.(38,39) Early defibrillation is the only effective therapy for pulseless ventricular tachycardia (pVT) and ventricular fibrillation (VF). We are unable to recommend the use of public access defibrillators for neonates owing to the extremely low incidence of shockable rhythms in the neonatal population and the lack of published validation on the accuracy of AEDs to analyse electrocardiographic algorithms in this age group.
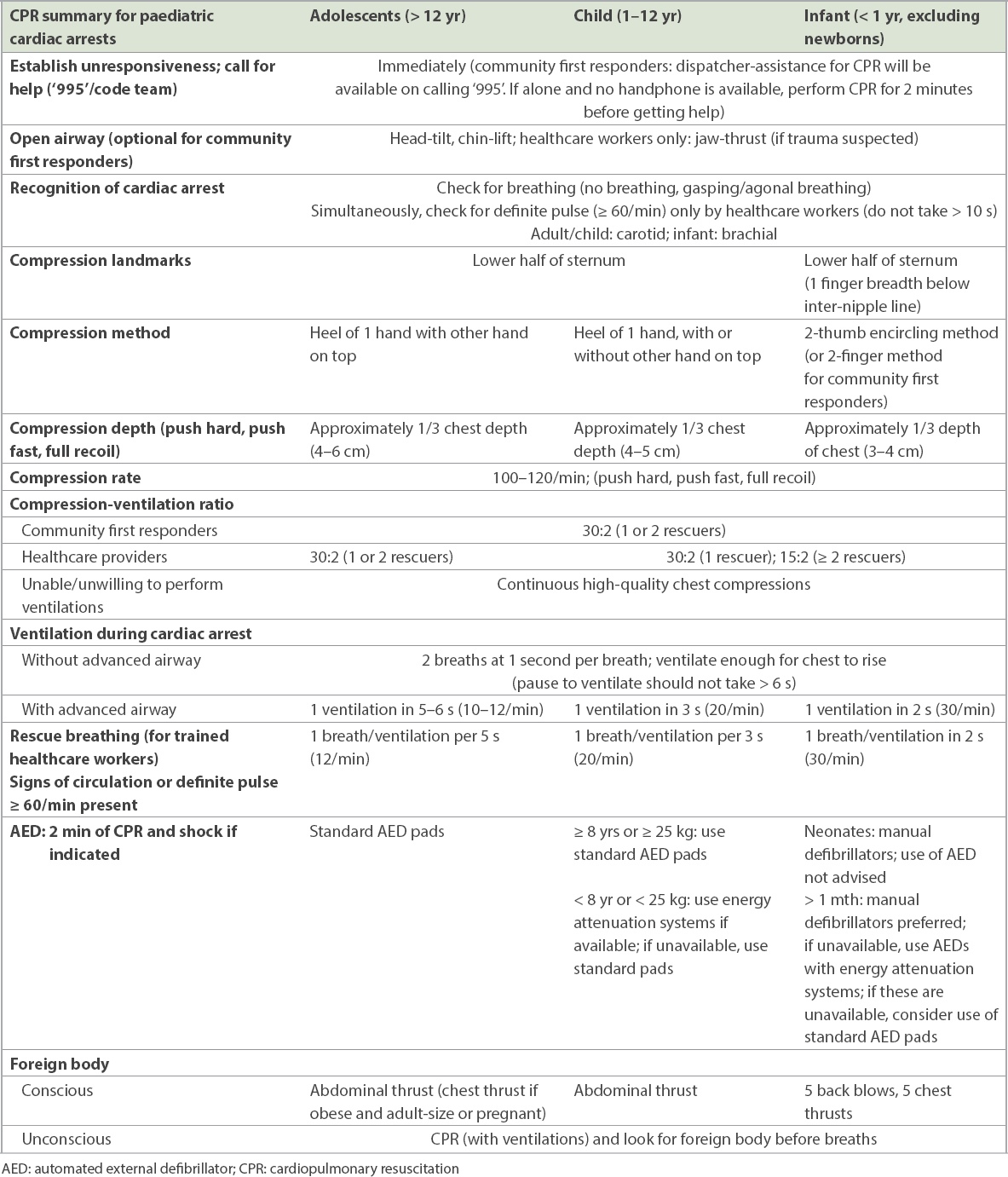
The subcommittee also highly recommends that schools, sports facilities and public areas be equipped with AEDs and, optimally, have attenuated energy systems (AEDs with paediatric modes or child pads) to cater to younger paediatric patients. A summary of the paediatric life support algorithm is shown in
Table I
Summary for Paediatric Life Support.
4. ADVANCED PAEDIATRIC LIFE SUPPORT
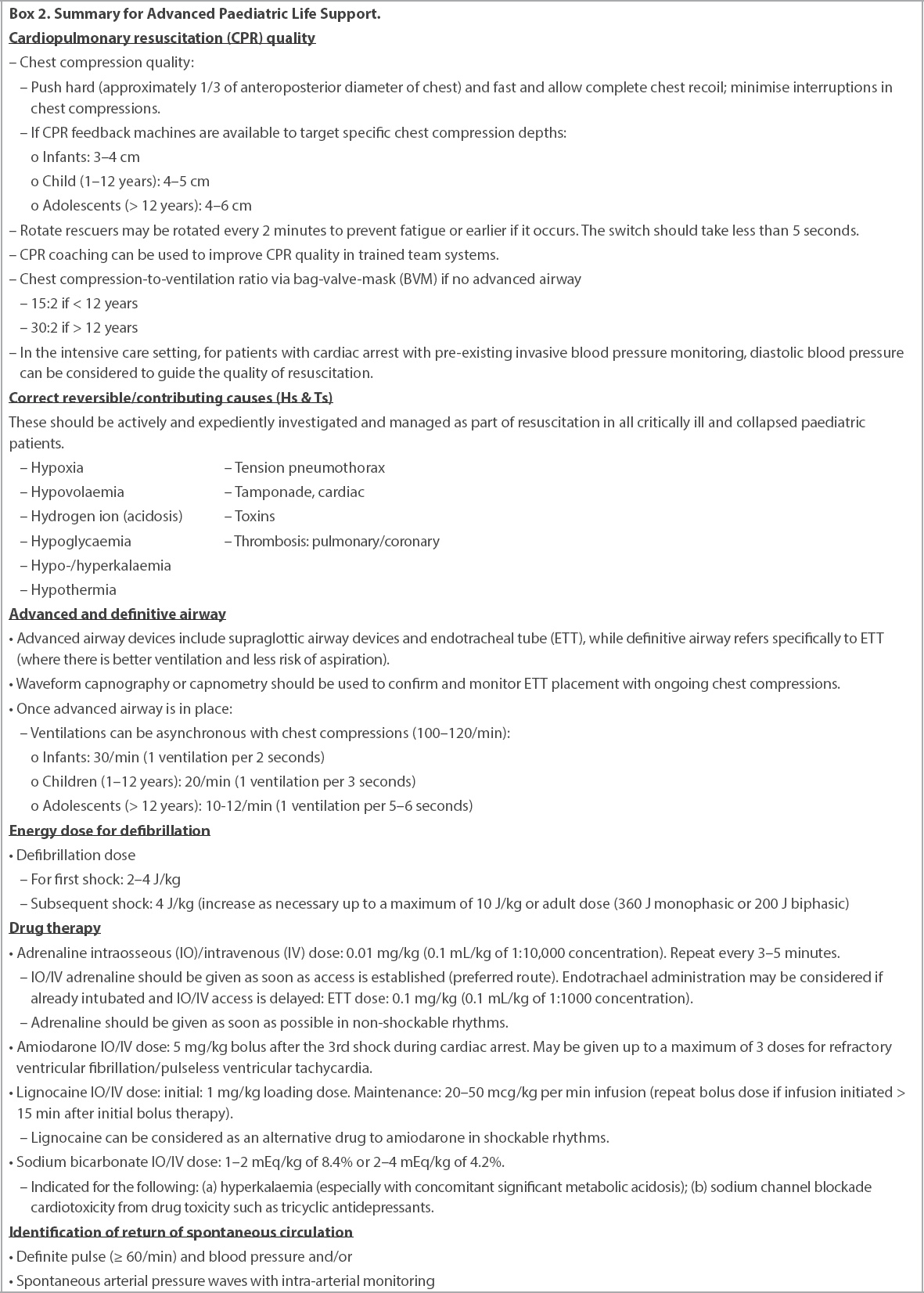
Box 2
Summary for Advanced Paediatric Life Support.
Ventilation rates post-advanced airway placement
Rationale and evidence update
Sutton et al demonstrated improved survival in intubated paediatric patients with higher ventilation rates during cardiac arrest in a small observational critical care study.(40) An observational pre-hospital adult cardiac arrest study showed no advantage in terms of survival and long-term neurological outcome for those who were ventilated < 10 breaths/min compared to those who were ventilated > 10 breaths/min (mean 15 breaths/min).(41)
However, concern remains regarding compromising venous return when patients with cardiac arrest are being hyperventilated (age-specific) when receiving positive pressure ventilation. Therefore, to avoid hypoventilating and hyperventilating paediatric patients during cardiac arrest, the subcommittee recommends ventilation at the lower age-specific normal respiratory range of ventilation rates, which are similar to those for assisted ventilation in paediatric patients who have respiratory insufficiency, but a perfusing rhythm and a pulse rate of > 60/min (rescue breathing).
New recommendation
After advanced airway placement in patients with cardiac arrest, it is reasonable to asynchronously ventilate infants and children undergoing chest compressions with the following – infants: 30/min (one breath in two seconds); child (1–12 years): 20/min (one breath in three seconds); and adolescents (> 12 years): 10–12/min (one breath in 5–6 seconds, ten compressions to one breath). Note that rates exceeding these recommendations may potentially compromise haemodynamics. In rescue breathing and assisted ventilation (healthcare providers) for respiratory insufficiency with a palpable pulse > 60/min (similar to intra-arrest), it is reasonable to use the following – infants: 30/min (one breath in two seconds); child (1–12 years): 20/min (one breath in three seconds); and adolescents (> 12 years): 12/min (one breath in five seconds).
Laryngeal mask airway and supraglottic device in pre-hospital setting
Effective airway management is fundamental in paediatric resuscitation, as most paediatric cardiac arrests are due to respiratory deterioration.(42) In the 2016 Paediatric Resuscitation Guidelines from the National Resuscitation Council, for pre-hospital paediatric resuscitation, it was recommended that bag-valve-mask ventilation (BVM) be used as initial management.(43) A paediatric meta-analysis suggested that BVM ventilation is not inferior to advanced airway interventions, and it is, therefore, reasonable to use BVM ventilation as initial management, especially if transport time is short. However, if there are issues with the ventilation of collapsed patients, supraglottic airway device may be used in conjunction with BVM.(6,44)
Paediatric cardiac arrest: non-shockable rhythms
We recommend that reversible causes should be evaluated for all paediatric patients with cardiac arrest. It is reasonable to use standard-dose adrenaline for paediatric cardiac arrest management. A systematic review and meta-analysis demonstrated improved outcomes when adrenaline is given as early as possible to paediatric patients with cardiac arrest with non-shockable rhythms.(45) Adrenaline should be given as soon as vascular access is established, following which adrenaline should be given every 3–5 minutes as needed, with regular evaluation. The resuscitation algorithm for paediatric cardiac arrest with non-shockable rhythms is shown in
Fig. 4
Flowchart shows the resuscitation algorithm for pulseless arrest. CPR: cardiopulmonary resuscitation; HR: heart rate; IO: intraosseous; IV: intravenous; PEA: pulseless electrical activity; q: every; ROSC: return of spontaneous circulation; VF: ventricular fibrillation; VT: ventricular tachycardia
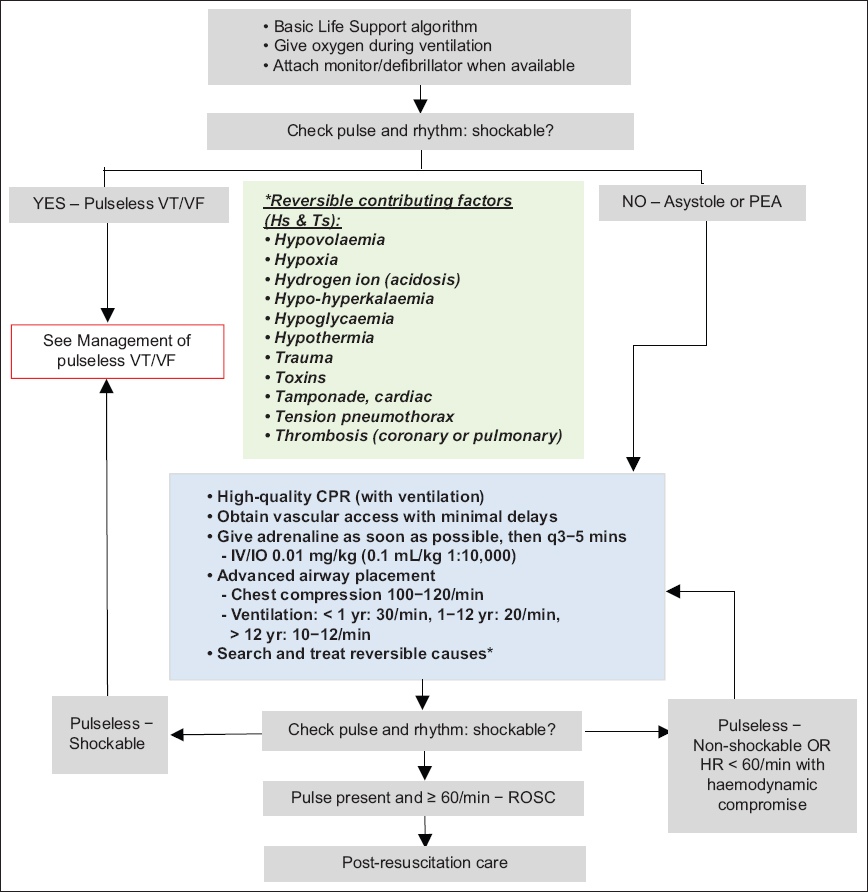
Paediatric cardiac arrest: shockable rhythms
The summary for management of paediatric cardiac arrest with shockable rhythms is shown in
Fig. 5
Flowchart shows the resuscitation algorithm for pulseless arrest with shockable rhythms. AED: automated external defibrillator; BVM: bag-valve- mask; CPR: cardiopulmonary resuscitation; IO: intraosseous; IV: intravenous; PEA: pulseless electrical activity; q: every; ROSC: return of spontaneous circulation; VF: ventricular fibrillation; VT: ventricular tachycardia
Energy dose for first defibrillation
We have updated the energy dose for initial defibrillation. A small observational study in the intensive care setting provided limited evidence for the initial energy dose for defibrillation to be lower than 4 J/kg.(46) However, there were significant limitations in the external validity of this single study. Given that the evidence is equivocal, we suggest that it is reasonable that the initial energy dose for defibrillation to be in the range of 2–4 J/kg. Units that have been trained to use 4 J/kg for the initial defibrillation should continue this practice.
New recommendation
It is reasonable to use an initial energy dose of 2–4 J/kg of monophasic or biphasic energy for defibrillation. If refractory, it is reasonable to consider using ≥ 4 J/kg energy levels, not exceeding 10 J/kg or the adult maximum dose, with a maximum of 360 J (monophasic) or 200 J (biphasic).
Anti-arrhythmic medications
For shock-refractory VF or pVT, either amiodarone or lignocaine may be used.(47) Amiodarone (5 mg/kg; maximum dose of 300 mg) can be administered intravenously or intraosseously in refractory VF or pVT at the third shock and at every alternate shock (up to a maximum of three doses). Lignocaine may be considered as an alternative to amiodarone in refractory VF. If lignocaine is used, an initial bolus of 1 mg/kg (maximum dose of 100 mg) may be given at the third or fifth shock, followed by a maintenance infusion of 20–50 mcg/kg/min. If there is a delay between the initial bolus and the start of the infusion by more than 15 minutes, another bolus of 1 mg/kg can be given.
The clinician should choose the drug that they are familiar with and for which they have knowledge of the drug’s side effects. We suggest that it is reasonable to administer amiodarone or lignocaine for the management of refractory VF or pVT.
Other cardiac arrest medications
A recent systematic review and meta-analysis on the empirical use of sodium bicarbonate in paediatric cardiac arrest showed that sodium bicarbonate did not result in improved outcomes except in special resuscitation situations such as hyperkalaemia (especially with concomitant metabolic acidosis) and sodium channel blockade cardiotoxicity from drug toxicity such as tricyclic antidepressants.(48)
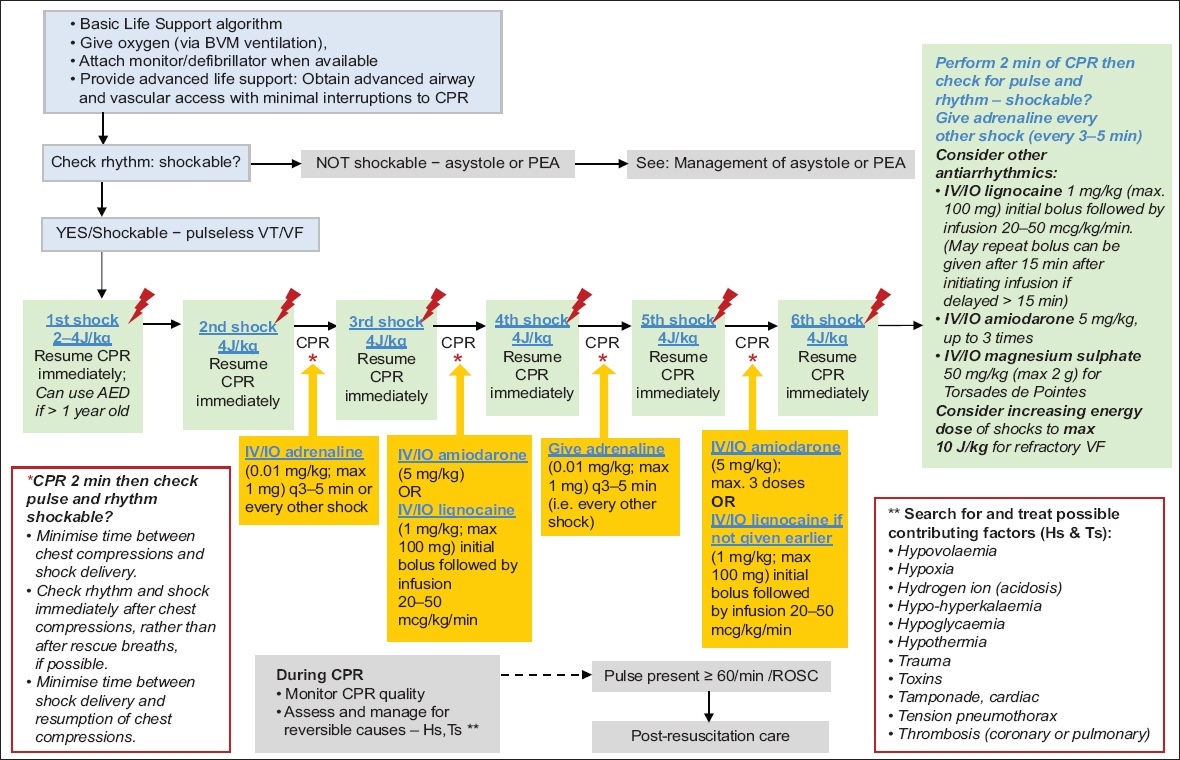
Bradyarrhythmias
Non-cardiogenic causes of bradyarrhythmias should be considered when assessing paediatric patients. In patients with asymptomatic bradycardia, consider the clinical context and non-cardiogenic causes. Patients with haemodynamic compromise with bradycardia may precede cardiac arrest. Ventilation and chest compressions should be initiated and reassessment made after intervention.
Fig. 6
Flowchart shows the algorithm for bradycardia. AV: atrioventricular; CPR: cardiopulmonary resuscitation; ECG: electrocardiogram; HR: heart rate; IO: intraosseous; IV: intravenous
Tachyarrhythmias
In tachyarrhythmias, assessment of the haemodynamic status of the patient is important. The management of tachyarrhythmias is shown in Figs.
Fig. 7
Flowchart shows the algorithm for stable tachycardia. ECG: electrocardiogram; HR: heart rate; IV: intravenous; SVT: supraventricular tachycardia
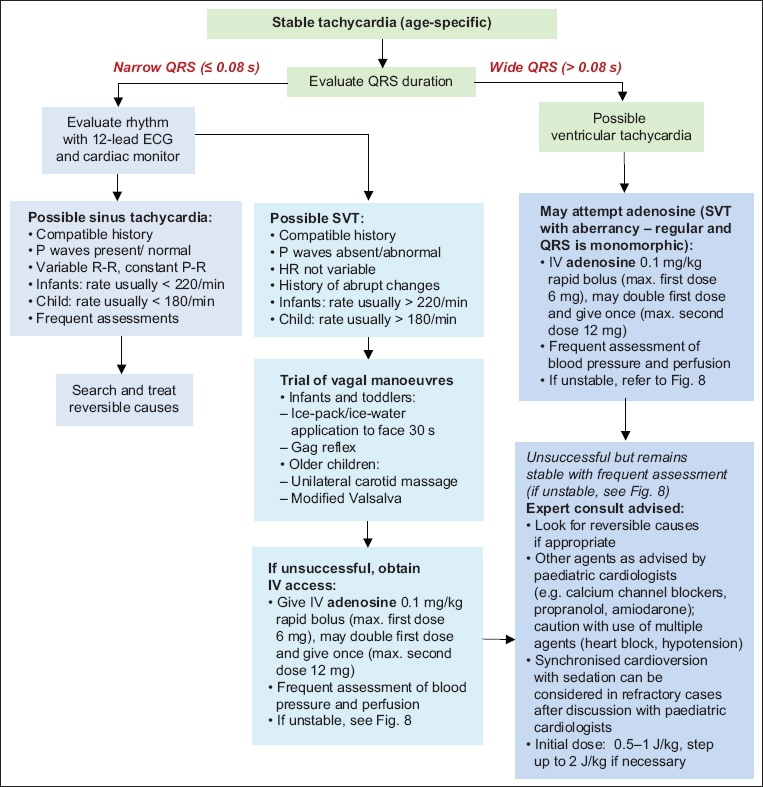
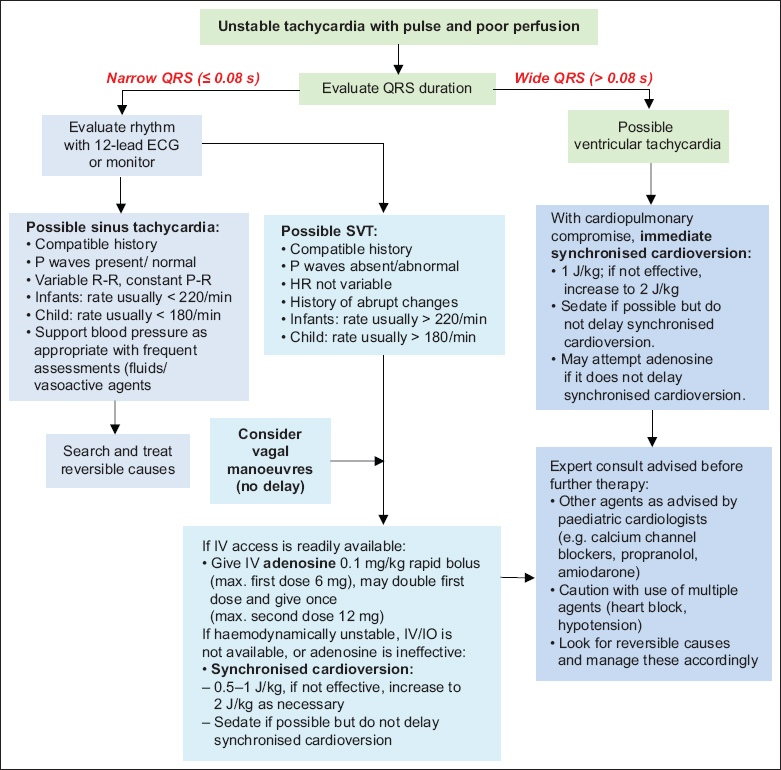
Fig. 8
Flowchart shows the algorithm for tachycardia with poor perfusion. ECG: electrocardiogram; HR: heart rate; IO: intraosseous; IV: intravenous; SVT: supraventricular tachycardia
Extracorporeal cardiopulmonary resuscitation for paediatric in-hospital cardiac arrest
There are no changes from the 2016 guidelines on the recommendations for the use of extracorporeal membrane oxygenation (ECMO) cardiopulmonary resuscitation (ECPR) in the paediatric population. ECPR may be considered for paediatric patients with cardiac arrest with underlying cardiac disease in in-hospital settings that have paediatric intensive care units with established expertise, resources and systems to optimise the use of ECMO during and after resuscitation.(49)
Intra-arrest prognostic factors: focused echocardiography, ETCO2 and invasive blood pressure monitoring
There is limited and conflicting scientific evidence regarding the use of bedside ultrasonography to prognosticate outcomes in paediatric cardiac arrest.(27) Similarly, the evidence for the use of end-tidal carbon dioxide (ETCO2) to prognosticate outcomes is extremely limited. Thus, the use of ETCO2 or bedside ultrasonography as prognostic tools during a paediatric cardiac arrest is considered largely investigational. A small observational paediatric study has found an association between diastolic blood pressure (DBP) targets and improved survival to discharge – infants: DBP > 25 mmHg; 1–12 years: DBP > 30 mmHg; and > 12 years: DBP > 35 mmHg.(50)
It is, thus, reasonable to suggest that for paediatric patients with pre-existing invasive arterial blood pressure monitoring who are in cardiac arrest, the use of DBP to guide and optimise resuscitative efforts may be considered.
Team training for Advanced Paediatric Life Support
Multidisciplinary team training, especially in high-acuity areas, is encouraged. Training and updates in paediatric life support knowledge and skills allow for location and function-specific needs to be considered during paediatric life support. Training strategies, such as using simulation to improve team dynamics, workflows and resources in anticipation, are encouraged. Such team training has been reported to translate to improved resuscitation performance.(51-53)
Family presence during paediatric cardiac arrest resuscitation
A recent systematic review on family presence during paediatric cardiac arrest resuscitation demonstrated potential benefits for the child’s parents if they are allowed to be present during the resuscitation.(54) However, these parents should be guided throughout the process by trained personnel. Familiarisation and training for health providers to facilitate parental presence in such high stress situations should be considered. Other considerations include infectious control measures during pandemics, which would limit the number of personnel in the resuscitation room for paediatric patients with cardiac arrest with suspected highly infectious agents such as the SARS-CoV-2 virus.(55)
New recommendation
It is reasonable to allow the patient’s family to be present during resuscitation, if there are dedicated and trained healthcare providers to guide them through the process and if family presence does not interfere with the resuscitation process and infection control measures.
5. POST-ARREST CARE IN PAEDIATRICS
There are no major changes on post-arrest care in paediatrics from the 2016 resuscitation guidelines.
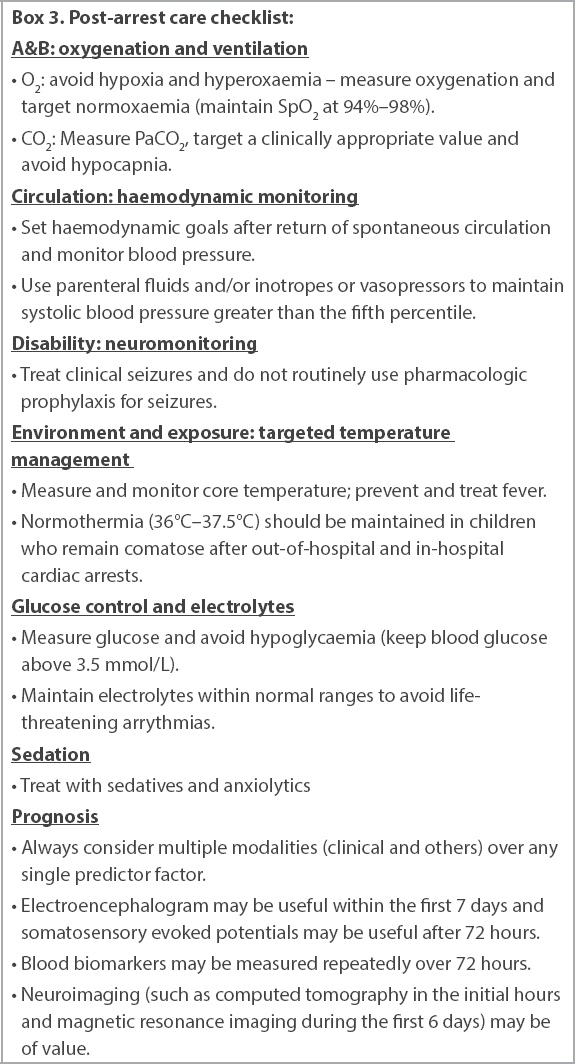
Box 3
Post-arrest care checklist:
Return of spontaneous circulation ventilation rates, PO2 and PCO2 targets
There are concerns about hyperoxia and hypoxia, as well as hyper- and hypoventilation after return of spontaneous circulation (ROSC).(56) Once there is sustained ROSC, we suggest that initial ventilatory rates can be targeted to allow for age-appropriate normoventilation – > 12 years: 12 breaths/min; 1–12 years: 20 breaths/min; and < 1 year: 30 breaths/min. In the absence of specific data on the patient and condition, it is reasonable to target normoxaemia and normocarbia after ROSC. It is also reasonable to use pulse oximetry after ROSC and to aim for an oxygen saturation of 94%–98%, or values deemed appropriate for patients with underlying chronic cardiac or pulmonary diseases.
Post-ROSC fluid and inotrope therapy
It is reasonable to maintain normal blood pressure that is appropriate for the age of the child after ROSC through the use of vasoactive agents or appropriate fluid management to ensure normotension.
Post-cardiac arrest targeted temperature management
We continue to recommend that normothermia (36°C–37.5°C) be maintained in children who remain comatose after OHCA and after IHCA. Hyperthermia should be prevented and actively managed if it occurs.(60)
Post-cardiac arrest seizure monitoring and management
Continuous electroencephalogram in the first week after arrest allows for better management of seizures, which can be subclinical. All seizures should be managed expediently.(61-63)
6. RECOVERY AND REHABILITATION
There is a need to ensure that paediatric cardiac casualties and their families are followed up beyond their hospitalisation period. An International Liaison Committee on Resuscitation workgroup looked at patient- and family-centric resuscitation outcomes to ensure that resuscitation guidelines and recommendations are targeted at these groups.(9)
As the emphasis shifts towards neurologically intact survival, rehabilitation and follow-up are needed to ensure optimal outcomes for the neuro-behavioural, functional and emotional well-being of paediatric cardiac arrest survivors and their families. Rehabilitation does not need to be limited to functional and physical therapy for those with more severe neurological sequelae after cardiac arrest. Those with mild or moderate neurological sequelae may benefit from neurological and/or functional rehabilitation to optimise their potential, owing to the plasticity of the paediatric brain. However, one of the current gaps is the lack of robust measurement of long-term neurological outcomes to guide our understanding of interventions and new guidelines.
Psychological support should be offered to all paediatric survivors of cardiac arrest and their families. In addition, there is a need to provide emotional or social support and follow-up for families of non-survivors of paediatric cardiac arrest.
CONCLUSION
A summary of the 2021 recommendations of the paediatric resuscitation workgroup is shown in the
ACKNOWLEDGEMENTS
The authors thank Prof Lim Swee Han, Chairman of Singapore Resuscitation and First Aid Council (SRFAC), and Mr Loke Jun Hao, Executive Member of SRFAC, for their invaluable input during workgroup discussions and assistance in the preparation of the recommendations.
Supplementary Material
SMJ-62-389.pdf



