Abstract
INTRODUCTION
Lead poisoning has been receiving great attention around the world. The Child Hygiene Cooperation Center of the World Health Organization in China has been conducting investigations to monitor blood lead levels (BLLs) from as early as 2004. However, only several lead poisoning studies have been conducted in China since August 2009. The aim of the present study was to investigate the BLLs in children aged < 7 years and to analyse the risk factors of high BLLs in Chengdu, China.
METHODS
Questionnaires were distributed to children in Chengdu from 2010 to 2011. A total of 2,271 children were included in this study – 1,157 received BLL tests in 2010 and the remaining received the tests in 2011. BLL was measured using a tungsten atomiser absorption spectrophotometer.
RESULTS
The mean BLL of the 2,271 children was 6.2 µg/dL and 2.03% of the children had BLLs ≥ 10 µg/dL. Mean BLL seemed to increase with age. Unhygienic habits (e.g. not washing hands frequently, biting of toys and pencils), history of pica, use of coal and residence in an industrial zone were found to be the main risk factors contributing to high BLL (p < 0.05). Children with high BLLs have a higher risk of manifesting anorexia and/or abdominal pain as compared to those with low BLLs (p < 0.05).
CONCLUSION
The mean BLL of children in Chengdu (i.e. 6.2 µg/dL) was found to be higher than that of children in developed countries. Childhood lead poisoning remains a public health problem.
INTRODUCTION
Lead, a highly toxic metal, is commonly found in the environment due to human activities, such as mining, manufacturing and burning of fossil fuels.(1) Lead is also widely used in a variety of products (e.g. lead car batteries, paints, water pipes, cosmetic products, hair dyes and building materials).(1,2) Therefore, the effect of lead exposure is an important health issue. This is especially the case when children are concerned, as they are more likely to play in dirt and insert objects into their mouths, resulting in a higher risk of lead exposure as compared to adults.(3) Many studies have revealed that elevated blood lead levels (BLLs), i.e. BLLs ≥ 10 µg/dL, can result in adverse health effects including encephalopathy, cardiovascular disease, immune system disease and anaemia.(4-10) Lead primarily affects the central nervous system, particularly the development of the brain.(1) Even low levels of lead exposure have been shown to be associated with impairment of children’s cognitive function and abnormal infant behaviour.(11-13)
Since the last half of the 20th century, lead poisoning has been receiving serious attention in developed countries.(14) Thereafter, regulatory and environmental reforms have occurred, which resulted in significant mitigation of elevated BLLs among children in developed countries.(15) In recent years, lead exposure has received serious attention from Chinese scientists, and since 2004, the Child Hygiene Cooperation Center of the World Health Organization (WHO) in China has been carrying out investigations to monitor the BLLs of children from 14 Chinese cities.(16) One study conducted in China reported that lead poisoning has affected more than 4,000 children in several Chinese cities and that the BLLs of most of the affected children were > 25 µg/dL.(17) Such findings indicate that there is a need for large, population-based investigations to objectively assess BLLs and that practical guidelines for the prevention and treatment of childhood lead poisoning should be developed. Thus, the aim of the present study was to collect large amounts of data (i.e. BLLs and health points) on the children living in Chengdu, so as to examine the BLLs and analyse the risk factors and symptoms of lead poisoning.
METHODS
Children aged < 7 years were eligible for inclusion in the study. Only the results of the first set of BLL tests were used, and the child’s name and identification number as well as time of investigation were documented. Children with known severe diseases, such as acute abdomen caused by acute appendicitis, were excluded. The number of children included in the study in 2010 and 2011 were 1,157 and 1,135, respectively. As 21 children participated in both enrolments, the second set of data (i.e. the data taken in 2011) of these children was excluded from the analysis. In other words, all analyses were done based on data from 2,271 children.
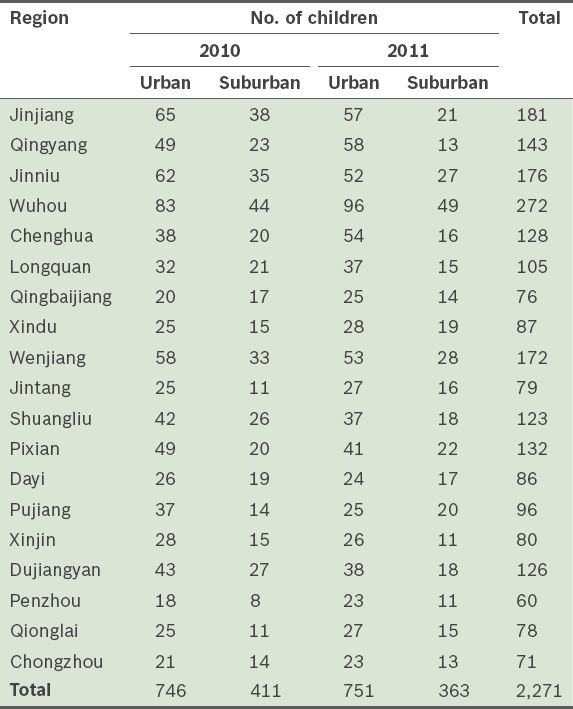
Children aged 3 to < 7 years were sampled from kindergartens during regular check-ups, while children aged < 3 years were sampled from community child health clinics during mandatory and periodic visits. Our study cohort included children from all 19 regions of Chengdu. The geographic distribution of the children is shown in
Table I
Geographic distribution of the children in the study (n = 2,271).
Venous blood specimens were collected in Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ, USA) containing heparin lithium, and BLL was analysed twice using a well-controlled BH2100 tungsten atomiser absorption spectrophotometer (Beijing Bohui Innovation Technology Co Ltd, Beijing, China) within an hour after sampling. The mean BLL of the two measurements was recorded. Seronorm™ Trace Elements Whole Blood (SERO, Billingstad, Norway) was used at the beginning and end of each day to ensure that the equipment was properly calibrated.
Data obtained from the questionnaires was inputted into an EpiData database (The EpiData Association, Odense, Denmark). In accordance with WHO guidelines, the present study defined elevated BLL as a BLL value ≥ 10 µg/dL.(18,19) Statistical analyses were conducted using the Statistical Package for the Social Sciences version 18.0 (SPSS Inc, Chicago, IL, USA). Variance analysis was used to examine the differences in BLLs between groups. Differences among the age, gender, nationality and housing environment of the children were evaluated in a pairwise method using t-tests. Logistic regression analysis was used to analyse the risk factors of high BLL. Chi-square test was used to assess the correlation between BLL and clinical symptoms. A p-value < 0.05 was considered to be statistically significant.
RESULTS
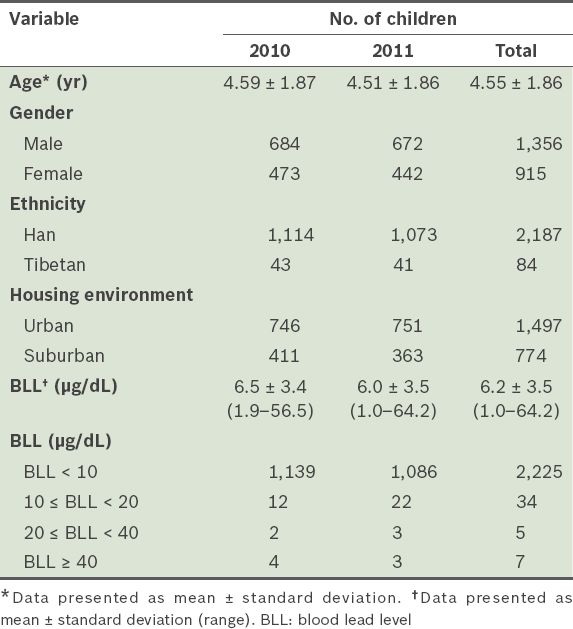
The mean age of the 2,271 children was 4.55 years (
Table II
Characteristics of the children in the study (n = 2,271).
The mean BLLs of the children according to their age are presented in
Table III
Mean blood lead levels (BLLs) and percentage of children (n = 2,271) with BLLs ≥ 10 µg/dL, according to age.
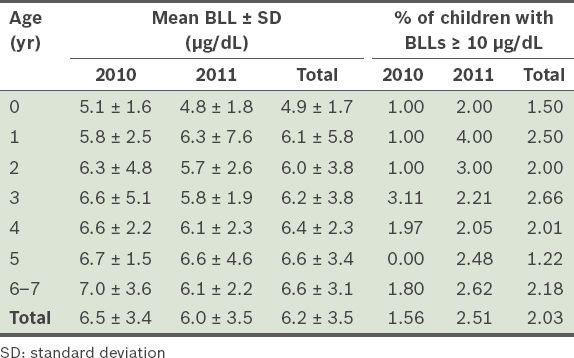
Fig. 1
Graph shows the trend of blood lead levels (BLLs) by age.
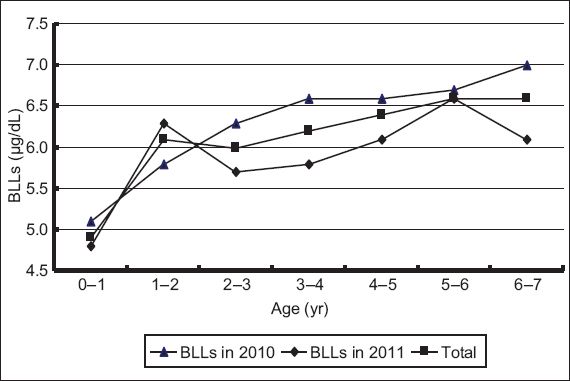
Table IV
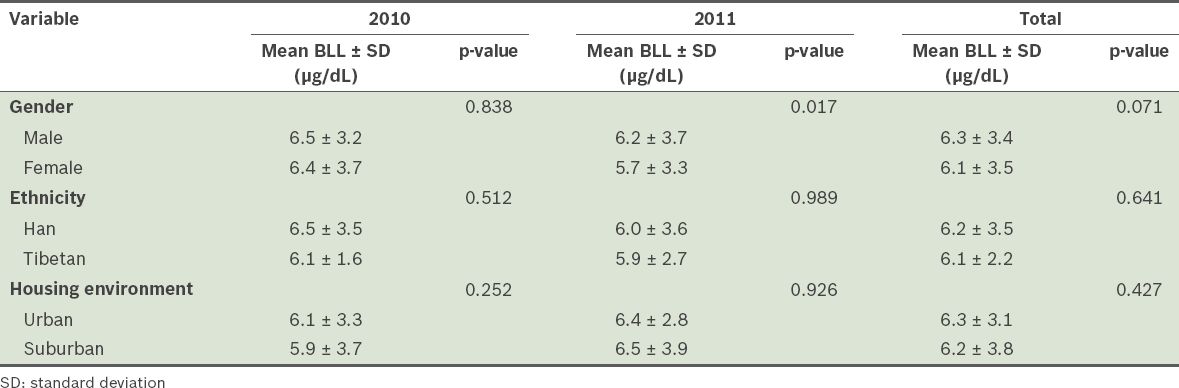
Mean blood lead levels (BLLs) of the children (n = 2,271) according to gender, ethnicity and housing environment.
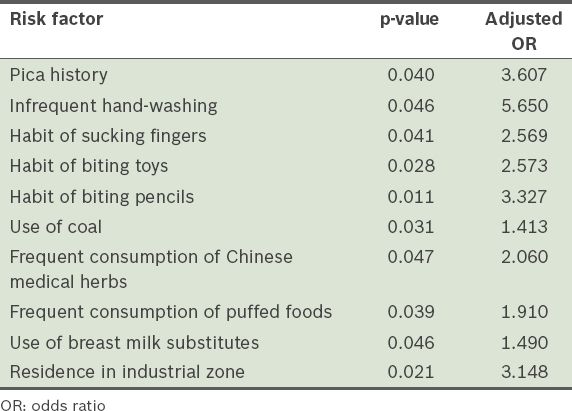
Logistic regression analysis was performed to find factors that were significantly associated with high BLL (i.e. ≥ 10 µg/dL). Confounders that were entered into the logistic regression analysis included gender (male), ethnicity (Han), living in scattered housing, residing in a single-storey house, residing in a house beside the streets, use of lead fuel and pica history. Factors that were found to be significantly associated with high BLL (i.e. p < 0.05) and their adjusted odds ratios are listed in
Table V
Risk factors found to be significantly associated with high blood lead level in logistic regression analysis.
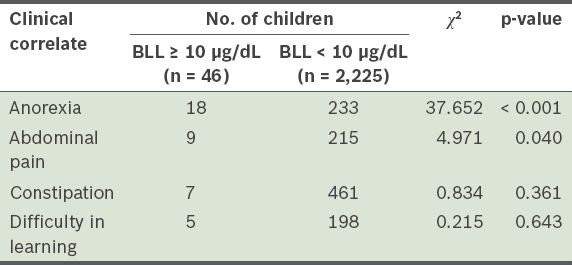
The correlation between elevated BLL and clinical symptoms such as anorexia, abdominal pain (i.e. lead-induced colic, and not acute appendicitis, cholecystitis or other causes of acute abdomen), constipation and difficulty in learning were assessed. The results of this analysis are shown in
Table VI
Clinical correlates of blood lead levels (BLLs) of children (n = 2,271) aged 0–7 years.
DISCUSSION
The mean BLL of the 2,271 children (aged < 7 years) in the present study was 6.2 µg/dL, and the prevalence of elevated BLL was found to be 2.03% for both years (2010 and 2011). We also observed that BLLs rose with increasing age. Several risk factors were identified to be associated with elevated BLL, including the use of coal, pica history, residence in an industrial zone, unhygienic habits (e.g. sucking fingers, not frequently washing hands, biting toys and biting pencils), the use of breast milk substitutes, and frequent consumption of Chinese medical herbs and puffed foods. The present study also showed that anorexia and abdominal pain were significantly associated with elevated BLL.
In the present study, the mean BLL of the children in 2010 and 2011 were 6.5 µg/dL and 6.0 µg/dL, respectively. In a previous study,(20) the mean BLL of children in Chengdu in 2007, 2008 and 2009 were found to be 6.6 µg/dL, 5.7 µg/dL and 6.4 µg/dL, respectively. In other words, the BLLs of children in 2010–2011 are similar to those in 2007–2009. One study reported that there was no difference in the BLLs of children before and after the lead poisoning event in Longchang, which is situated not far from the city of Chengdu.(17) As studies have shown that even ‘low’ BLLs (i.e. > 5 µg/dL) may influence children’s neurobehavioral performance,(1,21,22) effective measures should be taken to reduce the BLLs of children.
In 1997, the United States Centers for Disease Control and Prevention divided the prevalence of elevated BLL into three categories: (a) higher than 12%: high epidemic area; (b) 6%–12%: moderate epidemic area; and (c) lower than 5.9%: low epidemic area.(20) Based on this categorisation and the sharp decrease in the prevalence of elevated BLL (from 9.2%(19) to 2.03% since 2007) in Chengdu, Chengdu is now a low epidemic area. This sharp decrease in the presence of elevated BLL is likely due to the elimination of leaded gasoline, which has been implemented in China since July 1, 2000.
Although Chengdu currently belongs to the ‘low epidemic area’ category, the BLLs of the children in Chengdu are still relatively high; the mean BLL of the children in the present study was > 6 µg/dL. Therefore, even though no lead poisoning event has been reported in Chengdu (unlike in Longchang, which has BLLs > 25 µg/dL(17)), BLL detection should still be carried out routinely to prevent high BLLs from causing unwanted damages.
The present study’s finding that BLLs increase with age, from 0 to < 7 years, is consistent with that of many studies.(23,24) The evaluation of our 2011 dataset showed that BLLs were significantly higher in boys than in girls. This finding is consistent with that of Zhang et al’s study, which involved 14 Chinese cities in 2004–2006.(16) However, the 2007–2010 data from the present study and a previous study(20) yielded different results; the data obtained during that period showed no significant difference in the BLLs of boys and girls. This may be due to population mobility or sampling error. There may also be regional differences in the BLLs of boys and girls. In the present study, we also found that the mean BLL of Tibetan children was slightly lower than that of the Han children in Chengdu, although this difference did not reach a significant level.
In the present study, we found that children with unhygienic habits (e.g. sucking fingers, not frequently washing hands, biting toys and biting pencils) were more likely to have elevated BLLs. This finding, although not observed in our previous study,(20) is consistent with many other studies.(3,25-27) Frequent consumption of Chinese medical herbs and puffed foods as well as the use of breast milk substitutes were found to be risk factors for elevated BLL in both our previous study(20) and the present study.
Elevated BLL is a known multi-target toxicant, with effects on the haematopoietic, nervous, immune and gastrointestinal systems.(4-10) Abdominal pain is frequently reported when BLL is > 40 µg/dL. In the present study, anorexia and abdominal pain were found to be significantly associated with elevated BLL. While lead toxicity is an uncommon cause of acute abdominal pain, several cases of acute abdominal pain have been reported to be caused by lead toxicity.(28,29) The diagnosis of lead toxicity is often delayed, as the abdominal pain is often mistaken to be caused by acute appendicitis, acute cholecystitis or other more common causes of acute abdomen. The monitoring of BLLs in children suffering from anorexia and/or abdominal pain may be useful in identifying children with elevated BLL.
The present study was not without limitations. First, we only evaluated and identified a small number of risk factors associated with elevated BLL. Second, the sample size was relatively small. To obtain more comprehensive and accurate findings, future studies should evaluate a greater number of risk factors and use a larger sample size.
In conclusion, the BLLs of children in Chengdu were found to be higher than those of children from other areas in China and developed countries. As lead poisoning in children is a public health problem in China, unhygienic habits in children and environmental pollution should be considered and addressed. In order to prevent lead poisoning, there needs to be greater collaboration among the government, the society and families.


