Abstract
INTRODUCTION
The use of neuromuscular blocking agents (NMBAs) is common during general anaesthesia. Neuromuscular monitoring with a peripheral nerve stimulator (PNS) is essential to prevent postoperative residual neuromuscular block (PRNB), defined as a train-of-four (TOF) ratio < 0.9. PRNB remains a common complication and may contribute to morbidity in the postoperative anaesthetic care unit (PACU).
METHODS
An online survey was sent to anaesthesiologists in our department to assess their knowledge and clinical practices related to neuromuscular blockade. Next, a study was conducted on adult patients scheduled for elective surgery under general anaesthesia requiring NMBAs. Upon admission to the PACU, TOF monitoring was performed.
RESULTS
A large proportion of anaesthesiologists showed a lack of knowledge of neuromuscular blockade or non-adherence to the best clinical practices associated with it. The majority (98.7%) stated that they did not routinely use PNS monitoring. In the clinical study, TOF monitoring was only used in 17.9% of the 335 patients who were assessed. The prevalence of PRNB was 33.4% and was associated with the elderly (age ≥ 65 years), a higher dose of NMBA used, a shorter duration of surgery, and a shorter duration between the last dose of NMBA and measurement of PRNB in the PACU. The incidence of adverse symptoms in the PACU was observed to be higher in patients with PRNB.
CONCLUSION
PRNB remains a clinically significant problem, but routine PNS monitoring is rare in our institution. This is compounded by inadequate knowledge and poor adherence to best clinical guidelines related to neuromuscular blockade.
INTRODUCTION
The use of neuromuscular blocking agents (NMBAs) during anaesthesia is common. Neuromuscular monitoring with a peripheral nerve stimulator (PNS) is essential to prevent postoperative residual neuromuscular block (PRNB).(1) PRNB remains a common but usually undetected complication during the postoperative period. It occurs in 40%–60% of patients receiving intraoperative NMBAs(2-4) and is associated with increased morbidity, including pulmonary aspiration,(5) airway obstruction,(6) hypoxia(7,8) and even mortality.(9)
PRNB can be assessed with clinical tests (no longer recommended) and neuromuscular monitoring using a PNS.(1,10) The latter can be used to monitor the degree of and recovery from neuromuscular block. It is also used to guide the timing and dosage of reversal agent administration. There are various modes of monitoring, including the train-of-four (TOF) mode, which delivers four supramaximal electrical stimuli (T1, T2, T3 and T4) at 2 Hz, with an interval of at least 10 seconds between each TOF.(11) Qualitative TOF is determined by the number of twitches detected and/or the degree of fade (decreasing amplitude of successive twitches indicating PRNB) observed via visual or tactile means. However, these are not reliable compared with quantitative monitoring.(12-14) Quantitative TOF is the TOF ratio, or the ratio of the fourth (T4) to the first (T1) twitches, and is most commonly measured using an acceleromyography device. Recovery from paralysis after NMBA administration, defined as a TOF ratio at the adductor pollicis ≥ 0.9, can only be determined accurately with quantitative monitoring.(15) PRNB therefore occurs when the TOF ratio is < 0.9, which is associated with impaired pharyngeal function, increased risk of aspiration, weakness of upper airway muscles and airway obstruction.(16) PNS for assessing neuromuscular blockade is recommended for neuromuscular monitoring following NMBA administration during all stages of anaesthesia, and is best performed using a quantitative monitor.(1,17)
However, many anaesthesiologists fail to use or incorrectly use the TOF monitor, leading to an increased incidence of PRNB.(18,19) In Singapore, there is no data on the incidence of PRNB. Therefore, we initially conducted a survey among anaesthesiologists, and junior trainees on rotation, in our department to assess their knowledge of and clinical practices pertaining to PRNB. We subsequently performed a prospective observational study to establish the prevalence of and factors associated with PRNB in the postoperative anaesthetic care unit (PACU).
METHODS
For the anonymous online survey, we sought ethical approval from the SingHealth Centralised Institutional Review Board (CIRB reference 2016/3076), but it was exempted from CIRB approval. All specialist anaesthesiologists and trainees on rotation in our department were invited to voluntarily participate in the survey. The survey comprised 25 multiple-choice questions (Appendix 1). The initial questions examined (a) the demographics of the anaesthesiologists, while the others were related to PRNB, NMBA, reversal agents and neuromuscular monitoring: (b) training; (c) personal clinical preferences; and (d) assessing their knowledge of (Questions 13, 14, 16, 17, 19–23 and 25) and adherence to clinical best practices (Questions 2, 3, 6–8, 18 and 24) related to preventing PRNB.
The study protocol was approved by the hospital ethics committee (SingHealth CIRB reference 2016/2478) and was registered with ClinicalTrials.gov (ID NCT02930629). Adult patients (aged ≥ 21 years) who were scheduled for elective operation in the main operating theatre under general anaesthesia and who required tracheal intubation using NMBAs were screened and recruited in the pre-anaesthetic assessment clinic at Singapore General Hospital, Singapore. Surgical specialties in the main operating theatre did not include cardiology and urology. Day surgery theatres were excluded. Patients were excluded if it was not possible to apply TOF monitoring on the ulnar nerve area of their forearms or they had underlying neuromuscular disease. Written informed consent was obtained from all participants.
Patient characteristics such as age, gender, body mass index and American Society of Anesthesiologists (ASA) physical status score were recorded. Relevant perioperative data was recorded: timing, dosage and type of NMBA administered; duration of surgery and general anaesthesia; anaesthetic agent used for maintenance; and type and dose of reversal agents used.
Intraoperatively, the use of NMBAs and reversal agents was at the attending anaesthesiologist’s discretion. Immediately after the patients’ arrival at the PACU, PRNB was assessed with the use of a quantitative PNS (TOF-Watch® SX, Organon Inc, Ireland). Two electrocardiography electrodes were placed 4 cm apart over the ulnar nerve at the distal part of the patient’s wrist. A 50-mA TOF stimulus was applied, and the motor response of the thumb (adductor pollicis muscle) was measured to assess the degree of PRNB via the TOF count; when the TOF count was 4, the TOF ratio was measured. A second set of TOF results was then obtained. The final TOF ratio was the average of the two sets of TOF stimulations. If the discrepancy between the two initial readings was > 10%, two more TOF stimulations were applied,(18) and the average of the two closest measurements was calculated. Each set of TOF stimulations was taken 15 seconds apart. The presence of PRNB was also assessed by clinical tests (five-second hand grip test and five-second head lift test). The PACU nurses were asked to monitor the patients for any adverse incidents associated with PRNB (airway obstruction, desaturation and complaints of muscle weakness or blurred/double vision).
The primary outcome measure of our clinical study was the prevalence of PRNB on arrival in the PACU. The secondary outcome was the prevalence of PRNB-associated adverse symptoms in the PACU. Study variables that were recorded included: duration of surgery; administration and dosages of NMBAs and reversal agents; prevalence of intraoperative TOF monitoring; and temperature on arrival at the PACU.
For the survey, we examined the incidence of correct and incorrect answers to the knowledge- and best practice-based questions (Appendix 2). Incorrect answers were defined as those that were the wrong option or ‘Don’t know’. For the clinical study, based on an estimated prevalence of PRNB of 40%, a sample size of 305 participants would provide a precision level of 5.5%.(20) Results were expressed as numerical values and percentages for categorical variables, mean ± standard deviation for normally distributed continuous data, and median with interquartile range (Q1–Q3) for skewed continuous data. The total dose of NMBA administered was expressed as 95% effective dose mg per hour per kg (i.e. ED95 mg/kg/h).(21) The ED95 for atracurium and rocuronium is 0.2 mg/kg and 0.3 mg/kg, respectively.(22,23) Univariate analysis was performed. For categorical data, χ2 test or Fisher’s exact test were used. For continuous data, Student’s t-test was used. We further assessed the relationship between PRNB and potential risk factors using a logistic regression model including variables of clinical importance and those with p < 0.1 in the univariate analysis. Sensitivity, specificity and predictive values of the clinical qualitative tests were calculated according to standard formulae. All tests were two-tailed and a p-value < 0.05 was considered significant. Statistical analysis was performed with PASW Statistics version 18.0 (SPSS Inc, Chicago, IL, USA).
RESULTS
For the survey, we received a total of 150 out of 202 responses, a 74.3% response rate. The survey results can be found in Appendix 2. Incorrect answers ranged from 0% to 68.7%. 68.7% of the participants gave an incorrect answer when asked for the definition of NMBA duration of action, 45.3% did not know when the ideal time for neostigmine administration was, and 45.3% did not know the definition of when a patient is fully reversed. Non-adherence to best clinical practices was also variable (range 0%–98.7%). 98.7% did not routinely use PNS following NMBA administration, and 94.7% did not use TOF count during laparoscopic surgery and to guide reversal administration.
For the clinical study, 400 patients were enrolled after screening at the preoperative anaesthetic clinic in Singapore General Hospital, Singapore, from September 2016 to July 2017. Data was available for 335 patients (
Table I
Demographic data and clinical characteristics of the patients (n = 335).
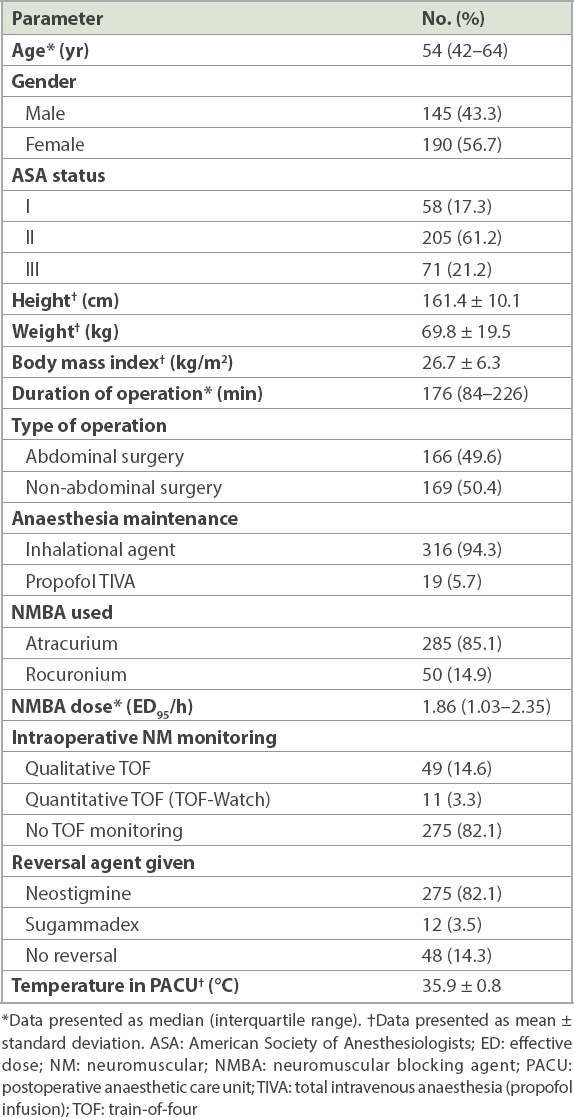
Table II
Adverse symptoms and incidents in the postoperative anaesthetic care unit.
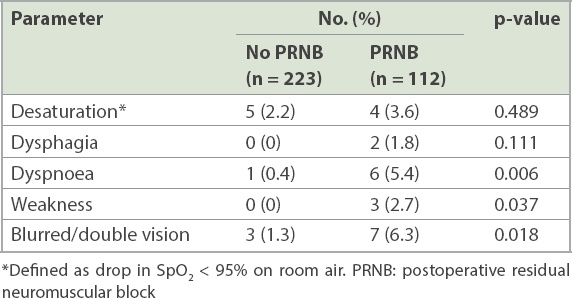
Univariate analysis showed no statistically significant differences in the prevalence of PRNB according to body mass index (< 30 kg/m2 vs. ≥ 30 kg/m2), ASA class, type of surgery, presence of hypothermia, type of anaesthetic/NMBA given or reversal agent given (
Table III
Risk factors associated with postoperative residual neuromuscular block (PRNB), assessed using univariate analysis.
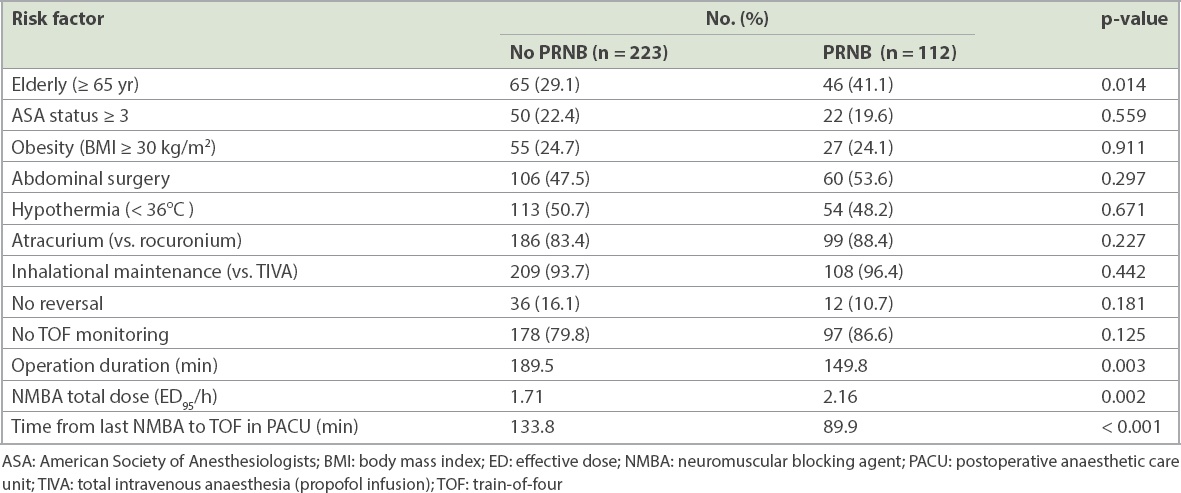
The prevalence of PRNB was associated with being elderly (≥ 65 years), a higher dose of NMBA used, shorter duration of surgery (149.8 minutes vs. 189.5 minutes) and a shorter duration between last dose of NMBA and TOF measurement in PACU (89.9 minutes vs. 133.8 minutes). Multivariate logistic regression showed that elderly status and a shorter duration between the last dose of NMBA and TOF measurement in the PACU were independent factors associated with PRNB (
Table IV
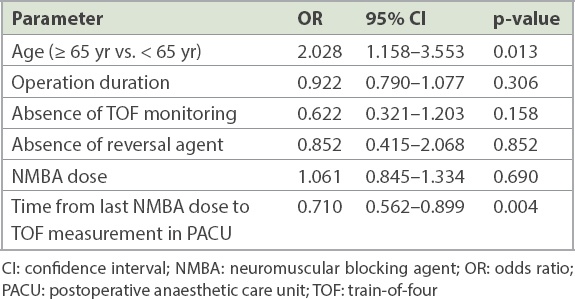
Determinants of postoperative residual neuromuscular block assessed using multivariate logistic regression.
During the TOF measurements, 85.4% of patients were easily roused and responsive to commands, falling into either the mildly or moderately sedated categories. Despite that, most patients tolerated the stimulation current of 50 mA well. Among the 335 patients, 15 (4.5%) reported no pain, 240 (71.6%) reported mild pain, and 77 (23.0%) reported moderate pain. Only 3 (0.9%) patients complained of severe pain during the TOF stimulations.
DISCUSSION
Our survey demonstrated that the rate of incorrect responses was high and that there was poor adherence to best clinical practices among anaesthesiologists. In the clinical study, the prevalence of PRNB in patients on their arrival at the PACU was 33.4%, and these patients had a significantly higher chance of having adverse symptoms. This is slightly less than in other reports,(2-4) which may be due to various factors: differences in definitions of PRNB (e.g. the diagnosing criteria for TOF ratio have changed since the 1970s), patient heterogeneity and the type of anaesthetic agents used. We herein discuss the results in relation to monitoring, reversal agents, duration of NMBA and risk factors for PRNB.
The use of PNS monitoring is recommended following administration of NMBA.(1) However, from our survey, only 32.0% of the respondents agreed and 6.0% strongly agreed with this recommendation. In a subsequent question, only 2.7% and 1.3% of our respondents stated that they used PNS monitoring in > 50% or in 100% of cases, respectively. This is comparable with results from a national survey of anaesthesiologists in Singapore by Teoh et al, in which only 13.1% of respondents said they monitored routinely.(24) In the clinical study, only 17.9% of our patients received any TOF monitoring intraoperatively, and only 3.3% had quantitative TOF monitoring.
The reason for the low rate of PNS monitoring use is multifactorial, including perception of the prevalence and consequences of PRNB use, PNS training, and availability of and confidence in using PNS monitoring. 34.0% of our respondents did not use PNS monitoring as they ‘don’t think it’s necessary’. This may be due to the perceived low incidence of PRNB. 24% of our respondents underestimated its incidence as 0%–19%. Similarly, 52% and 64% of those surveyed in Europe and the United States, respectively, stated that PRNB occurs in < 1%.(19) Similarly, in the survey by Teoh et al, 63.6% stated that it occurred in < 5%.(24) In the present survey, 94.0% and 62.7% of respondents stated that they had been taught TOF count and ratio, respectively. However, only 37.3% were confident enough to teach the use of the PNS. 8.7% stated that a lack of familiarity prevented them from using PNS monitoring. Lack of equipment (42.7%) was also cited by our respondents as a reason for not using PNS monitoring. However, another survey showed that even with widespread (95.8%) availability of monitors, only 13.1% would use them routinely and 60.3% would not change their practice of only sporadically monitoring their patients.(24)
The clinical signs that we studied showed poor sensitivity and specificity when used to assess for PRNB in the PACU, and are not recommended for determining recovery from neuromuscular block.(1,11) In addition, subjective assessments of TOF fade (equating to PRNB) are not reliable. Studies have shown that most operators are unable to detect fade if TOF ratio is ≥ 0.4.(12) Therefore, a TOF ratio of 0.4–0.9 gives rise to undetectable (minimal) fade, which has been termed the ‘zone of blind paralysis’.(25) As such, only quantitative monitoring can diagnose full neuromuscular recovery.
Interestingly, there was no significant difference in the prevalence of PRNB between the group who received monitoring and the group that was not monitored. A meta-analysis showed that intraoperative quantitative neuromuscular monitoring did not reduce the prevalence of PRNB.(21) This may be due to inappropriate use or misinterpretation of TOF monitoring, or inappropriate timing/dose of the reversal agent. With education and training as well as increased use of quantitative TOF monitoring and the use of a reversal agent, the incidence of PRNB can be significantly reduced (from 62% to 3%).(18) Acceleromyography has been shown to reduce postoperative hypoxaemia and improve quality of recovery in the postoperative period.(26)
The majority (85.7%) of our patients received pharmacological reversal at the end of surgery, with 82.1% of them receiving neostigmine. Studies have shown that when reversal is not given, there is a high incidence of PRNB of up to 42% (TOF < 0.7) to 45% (TOF < 0.9) of patients.(27) Also, a large retrospective study showed that lack of reversal was an independent risk factor for anaesthesia-related postoperative morbidity and mortality.(9) However, we did not find that the use of a reversal agent reduced the prevalence of PRNB. As mentioned, this may be due to inappropriate dose or timing of the reversal agent. Neostigmine for reversal should only be given when the TOF count is 4.(25) However, only 54.7% of our survey respondents agreed with this. 30.0% of the respondents followed the traditional criteria (TOF count of 2), which may lead to ineffective reversal and PRNB. In addition, when neostigmine is given to patients who have, or almost have, recovered from neuromuscular blockade, it can cause a paradoxical decrease in TOF ratio, muscle weakness and prolonged neuromuscular blockade.(28) This explains why quantitative (compared with qualitative) TOF monitoring is considered the best method,(1) as it helps guide the dose of reversal as well as when not to administer it.
With qualitative TOF monitoring, the dose can be titrated accordingly. 40 mcg/kg of neostigmine should be given to patients who demonstrate detectable fade.(1) In the absence of apparent (visual or tactile) fade (‘zone of blind paralysis’), 20 mcg/kg of neostigmine should be used instead.(25) This lower dose of neostigmine is effective in antagonising minimal neuromuscular blockade without significant paradoxical effects.(29) Even after administration of neostigmine, recommendations include a further wait of 10–15 minutes before waking and extubating the patient, to allow the peak effect of the drug to occur.(1,30)
Sugammadex binds specifically to steroidal NMBAs such as rocuronium and vecuronium.(11) Unlike neostigmine, it can reliably and rapidly reverse any degree of neuromuscular block due to rocuronium.(31) However, if sugammadex is used without neuromuscular monitoring, inappropriately administered dosages can lead to PRNB in up to 10% of patients.(32) In our study, sugammadex was rarely used (3.5%). Limiting factors for its use include its relatively high price, being centrally stored and the need to be prescribed on a named-patient basis.
There is a common misconception about the duration of action of NMBA that may lead to incorrect and dangerous clinical practices regarding the administration of NMBA and reversal agents. The duration of NMBA is often stated as the return of muscle twitch height (T1%) to 25%, while recovery is a return of TOF ratio ≥ 0.9. However, only 31.3% and 24.7% of our respondents, respectively, answered these correctly. It is clinically important to note that duration of action does not equate to recovery from the action of NMBA. The duration of action of a single intubating dose (2 × ED95) of intermediate-acting NMBAs is approximately 30–40 minutes. However, spontaneous recovery to TOF ratio ≥ 0.9 may take up to 2–6 hours.(4) Without proper understanding and monitoring of this difference, patients may be at risk of PRNB. After surgery lasting one hour and two hours, only 67.3% and 35.3% of our survey respondents, respectively, stated that they would always give reversal, while 6.7% said they would not usually give reversal after two hours.
Moreover, the duration of NMBA action and the risk of PRNB is increased by a variety of factors: large interpatient variability in the speed of spontaneous recovery; slower elimination in elderly patients; hypothermia; use of halogenated volatile anaesthetic agents; and cardiac, liver and renal dysfunction.(4) However, only 20.7% and 21.3% of our respondents stated that they would use PNS monitoring in patients with severe renal and liver disease, respectively. Infusions of NMBA also prolong its duration, but only half of our respondents used PNS monitoring in such cases.
Two independent factors were associated with PRNB in our study: age of the patient (≥ 65 years) and timing of the last dose of NMBA, which is in keeping with the current literature. The odds of patients aged ≥ 65 years having PRNB were double compared to the younger patient population. Murphy et al(33) also found a much higher incidence of PRNB in the elderly (age 70–90 years) compared with younger (18–50 years) patients (58% vs. 30%, respectively). This may be explained by elderly patients having altered pharmacokinetics and pharmacodynamics of NMBAs due to reduced physiological function and muscle mass, and the presence of multiple comorbidities. These decrease the clearance and increase the duration of action of NMBAs. Advanced age has also been identified as a risk factor for poor pulmonary outcome after NMBA use: one study showed that postoperative pulmonary complications occurred in 15% of its elderly patients but in only 2% of younger patients.(33) Elderly patients are at higher risk of these complications due to their poorer respiratory reserve: decreases in vital capacity, maximum voluntary ventilation and total lung capacity; and increases in functional residual capacity and closing volume. Elderly patients therefore require TOF monitoring and may need additional oxygen therapy, extra stimulation to maintain oxygenation and perioperative chest physiotherapy.
A shorter duration between the last dose of NMBA and TOF measurement on arrival in PACU was also associated with increased incidence of PRNB. This occurred although both NMBAs used in our study were intermediate-acting drugs. There are a few possible explanations for this. First, anaesthesiologists may not appreciate the high prevalence of PRNB or its consequences.(19) 24.0% of our survey respondents stated that the prevalence of PRNB is < 20%, while 29.3% said they did not know its prevalence. Second, as discussed, the action of even intermediate NMBAs may last longer than expected.(4) Regular top-ups or a last dose of NMBA given without TOF monitoring may result in undetected deep block. TOF monitoring was used in only 17.9% of the cases in our study. Third, there may have been a lack of TOF monitoring or an error in interpretation, resulting in inappropriate timing, dose and type of reversal agent used. Our survey showed that most anaesthesiologists either routinely give neostigmine reversal or administer it depending on the time of the last dose of NMBA; only 24% of respondents used PNS to guide the usage of reversal agents.
Another risk factor identified during our univariate analysis was the large total dose of NMBA used. Our survey showed that 68.7% of respondents incorrectly reported the definition of duration of action of NMBA, with 24.7% incorrectly stating that the duration was TOF ≥ 0.9 (i.e. recovery of block). This may lead to overdosage of NMBA and an increased risk of PRNB.
The aforementioned risk factors and the highly variable duration of action of NMBA makes it unsafe to exclude PRNB based on the time from the last dose of NMBA. Hence, proper use of PNS for neuromuscular monitoring is essential. Other factors that may potentially contribute to PRNB but were not statistically significant in our study include obesity, hypothermia and ASA 3. The lack of statistical significance may be because the majority of our patients were relatively fit and their temperatures were mostly normal (36°C).
Although the overall prevalence of adverse events was relatively low (1%–3%), it was significantly higher in the PRNB group. One patient (out of 335) with PRNB developed stridor requiring further dose of neostigmine in the PACU. In an institution such as ours with a high turnover of anaesthetised patients requiring NMBA, this may equate to a significant number of patients developing critical respiratory adverse events per year. A case control study by Murphy et al demonstrated that 73.8% of patients who developed critical respiratory events had a TOF < 0.7, which suggests that PRNB may be a contributing factor to the development of such events in the PACU.(26) Blurred vision and weakness can also cause considerable discomfort and may affect patient safety after transfer back to the ward. In our study, the prevalence may have been underestimated, as we did not examine adverse effects that could occur after discharge from the PACU, such as atelectasis, aspiration pneumonitis or increased length of hospital stay. We had only one case of stridor, but such airway obstruction may lead to laryngospasm and negative pressure pulmonary oedema.(34) With proper usage of NMBA monitoring and reversal, these events are mostly preventable and patient safety can be improved.
Our study was not without limitations, and the results should be interpreted in consideration of these. It was designed as a prospective observational study. The type of anaesthesia and monitoring used intraoperatively was left to the discretion of individual consultants and large practice variability was reflected in our findings. The study was only powered to identify the prevalence of PRNB, and hence was only able to generate associations for areas of further study. We did not aim to observe if the TOF monitoring was used to determine the optimal time for tracheal intubation, interval dosing of NMBA or appropriateness for extubation (i.e. when fully recovered).
Our study may underestimate the incidence of PRNB for various reasons. First, we used acceleromyographic TOF monitoring, which has been shown to give higher values of TOF ratio than the ‘gold standard’ mechanomyography.(35) It has been recommended that recovery to exclude PRNB should be redefined as acceleromyographic TOF ratio > 1.0.(10) Second, we used uncalibrated TOF ratio using 50 mA currents; this may not provide the necessary supramaximal stimulation required for TOF in some patients. Third, our study was confined to the PACU and we did not investigate the long-term effects of PRNB. Fourth, the site of TOF monitoring affects its interpretation. The muscle most commonly used to monitor TOF, the adductor pollicis, is less sensitive to NMBAs compared to the muscles of the tongue, floor of the mouth and the pharyngeal airway. Therefore, PRNB of the upper airway muscles could be present despite recovery of TOF ratio at the adductor pollicis. In our survey, 67.3% of the respondents gave incorrect answers regarding the order of resistance of various muscles to NMBAs. Although investigators were not blinded to the patient’s management regarding the use of NMBAs or reversal agents, we used an objective endpoint (i.e. TOF ratio).
As our overall risk of respiratory complications was low, we were unable to establish a firm association or causality between PRNB and the complications. The study was also conducted within a PACU setting with a second review at 24 hours. Therefore, we were unable to identify any long-term complications such as aspiration, pneumonia, prolonged hospital stays or other associated morbidity.
In conclusion, PRNB remains a clinically significant problem and rates of routine PNS monitoring are low in our institution. This is compounded by incorrect knowledge and inappropriate clinical practices related to neuromuscular blockade. Further education is needed to enhance the knowledge of anaesthesiologists. We also strongly encourage adherence to guidelines in the use of NMBAs and reversal agents as well as PNS monitoring. This is especially important in the elderly when high doses of NMBAs are used, after a shorter duration of surgery and when the last dose of NMBA is given close to the end of the surgery.


