Abstract
INTRODUCTION
Idiopathic nephrotic syndrome (INS) is the commonest type of nephrotic syndrome in children, and a majority of cases have favourable outcomes. A small proportion of INS cases progress to chronic kidney disease (CKD). We investigated the time to CKD and predictive risk factors associated with progression of CKD in these children.
METHODS
A retrospective review of medical records was done to investigate the demographic variables, and biochemical and histological changes in children with INS aged 12 months to 18 years between 2001 and 2016 at Hospital Universiti Sains Malaysia. The median renal survival time for progression to CKD stage III or higher was determined using survival curve analysis. Multiple Cox regression analysis was used to identify predictive factors for CKD.
RESULTS
The total number of participants was 112 (boys: n = 71; girls: n = 41) and a majority had steroid-sensitive INS. Only about 10% of INS progressed to CKD Stage III or higher, with an overall median renal survival time of 19 years. Median renal survival time in steroid-resistant nephrotic syndrome (SRNS) was 13 years. Focal segmental glomerulosclerosis was predominant in SRNS. The predictors of progression to CKD were steroid resistance (adjusted hazard ratio [HR] [95% confidence interval (CI)] 23.8 [2.8–200.9]) and the presence of hypertension at presentation (adjusted HR [95% CI] 8.1 [1.2–55.7]).
CONCLUSION
The median renal survival time in our study was comparable to other studies. SRNS and the presence of hypertension at presentation were the main predictors for developing CKD in our population.
INTRODUCTION
Idiopathic nephrotic syndrome (INS) is the commonest type of nephrotic syndrome in children. Worldwide incidence of INS has been reported to be up to 16 per 100,000 children.(1,2) The incidence is much higher among Asian populations (16.9 per 100,000 children) when compared to European and Afro-Caribbean (2.6–3.4 per 100,000 children) populations.(3) The condition is characterised by the presence of proteinuria, hypoalbuminaemia, oedema and hyperlipidaemia. INS is mainly a minimal change disease, which is steroid sensitive, accounting for 80%–90% of all cases. The initial response of patients to steroid therapy has been established as one of the important prognostic markers for children with INS.(4-6) Those with steroid-resistant nephrotic syndrome (SRNS) and confirmed focal segmental glomerulosclerosis (FSGS) carry the worst prognosis,(2,3) as approximately 64% of FSGS cases progress to end-stage renal disease (ESRD)(7) within five years of presentation.(8)
Renal biopsy is indicated for all children with SRNS, as recommended by Kidney Disease Improving Global Outcomes (KDIGO) guidelines in 2012.(9) However, this cannot be done for all patients due to parental refusal and, specifically so, among our study population. Due to limited histological diagnosis, other clinical and biochemical changes could guide us to determine who might be carrying the worst prognosis among children with INS. Apart from resistance to steroid therapy, older age at the onset of illness(3,8) and the presence of atypical features, namely microscopic haematuria, hypertension(8) and acute kidney injury (AKI)(2,10,11) at presentation, were predisposing factors towards the development of chronic kidney disease (CKD).
To the best of our knowledge, there is scanty data on the median renal survival time for progression to CKD and its predictors in Asian populations. The aim of this study was to describe the demographic profile and determine the median renal survival time, as well as the predictive factors to developing CKD Stage III or higher, among children with INS in Malaysia.
METHODS
This was a retrospective review of medical records, from 2001 to 2016, conducted at Hospital Universiti Sains Malaysia, Kelantan, Malaysia, which is a tertiary hospital in the northeast of Peninsular Malaysia. The study received ethical approval from the Human Research Ethical Committee Universiti Sains Malaysia. All children with INS, aged between 12 months and 18 years at the time of diagnosis, were enrolled in the study. Children diagnosed with infantile and secondary nephrotic syndromes, and those who were followed up for less than 12 months were excluded. Data analysed included age at presentation and last review, gender, ethnicity, weight, height, presence of hypertension, haematuria, AKI at presentation, serum creatinine level, histological changes and disease outcomes (e.g. response to steroids, death or ESRD).
The KDIGO 2012 guidelines were used as a reference to define clinical nephrotic syndrome, remission, relapse, steroid-sensitive and steroid-resistant type INS, haematuria, and AKI.(9) The treatment protocol was also based on KDIGO recommendations.(9) Hypertension was defined as blood pressure of more than the 95th percentile for age, weight and height for more than three consecutive readings,(12,13) and this was determined before initiation of steroid therapy. CKD was diagnosed when the estimated glomerular filtration rate (eGFR) was less than 60 mL/1.73 m2/min (CKD Stage III and above).(14)
eGFR was calculated based on the modified Schwartz formula: eGFR = 40 × height (cm)/serum creatinine (μmol/L), using the latest documented serum creatinine level. eGFR < 60 mL/1.73 m2/min (CKD Stage III and above) for more than three months was considered significant, as it is associated with complications such as altered drug excretion, impaired exocrine and metabolic function of the kidney, as well as higher risk of cardiovascular disease and death.(14)
Data was analysed using IBM SPSS Statistics version 22 (IBM Corp, Armonk, NY, USA). Continuous data was expressed as mean ± standard deviation, while discrete data was expressed as numbers and percentages. Kaplan-Meier analysis was used to estimate median renal survival time. Median renal survival time was measured from the date of diagnosis to the time of developing CKD Stage III or higher in years. Simple Cox regression analysis was conducted and factors with p < 0.25 were selected as potential predictive factors. Multiple Cox regression analysis was performed to identify the risk factors for CKD stage III or higher. Statistical significance was set at p < 0.05.
RESULTS
A total of 112 patients were enrolled. There were 71 (63.4%) boys and 41 (36.6%) girls. Most patients were of Malay ethnicity (n = 110, 98.2%) while only 1 (0.9%) patient was Chinese and 1 (0.9%) patient was Siamese. Mean age at initial diagnosis was 5.0 ± 3.0 years and duration of follow-up was 6.6 ± 3.8 years. The overall median renal survival time was 19 years. The proportions of steroid-sensitive nephrotic syndrome (SSNS) and SRNS were 72.3% (n = 81) and 27.7% (n = 31), respectively. At presentation, 11 (9.8%) patients had microscopic haematuria, 15 (13.4%) patients had hypertension and 6 (5.4%) patients had AKI. There were 12 (10.7%) patients who developed CKD – 11 SRNS and one steroid-dependent or frequently relapsing nephrotic syndrome (SDNS). 6 (5.4%) patients progressed to ESRD – four patients continued to be on chronic dialysis while two died.
There were 32 patients who underwent renal biopsy. Among these, 27 (84.4%) patients had SRNS whereas the remaining 5 (15.6%) patients had SDNS. Histological findings were as follows: 14 (43.8%) FSGS, 11 (34.4%) minimal change disease and 7 (21.9%) mesangioproliferative glomerulonephritis. Among the 12 (10.7%) patients with CKD, FSGS was confirmed for 5 (41.7%) patients, minimal change disease in 1 (8.3%) patient and mesangioproliferative glomerulonephritis in 1 (8.3%) patient. The histological diagnoses were not obtained for 5 (41.7%) patients with CKD due to parental refusal of renal biopsy.
Out of 81 patients with SSNS, 16 (19.8%) patients had SDNS, requiring additional immunosuppressive agents. Three of these patients continued to relapse into adulthood, but only one progressed to CKD.
In this study, 43 patients required second-line treatment – 25 patients with SRNS and 18 patients with SSNS – who were either steroid dependent, frequent relapsers or complicated with steroid toxicity. Cyclosporine was used for 27 patients – in 22 patients as a second-line agent and in five patients as the third-line agent. Among all patients on cyclosporine, 16 (59.3%) patients achieved remission. Out of eight patients on cyclophosphamide, 3 (37.5%) achieved remission. Two SRNS patients became steroid sensitive after receiving second-line immunosuppressive therapy.
The median renal survival time for SRNS was 13 years (
Fig. 1
Kaplan-Meier plot shows time to renal survival in children with idiopathic nephrotic syndrome (maximum follow-up time = 17 years).
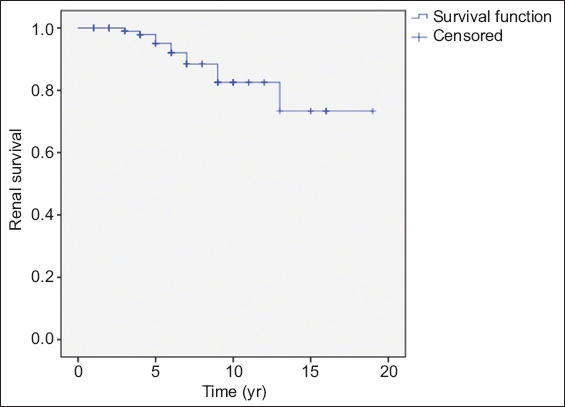
Table I
Simple Cox regression analysis of potential factors for developing CKD Stage III and higher in children with idiopathic nephrotic syndrome.
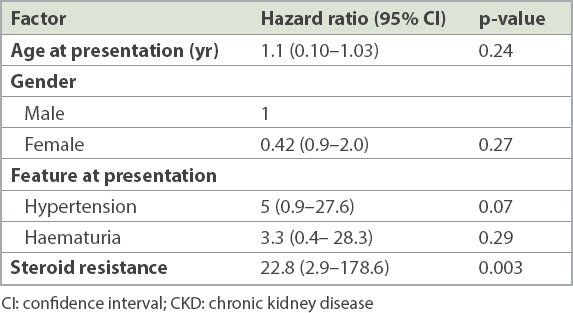
Table II
Multiple Cox regression analysis of potential factors for developing CKD Stage III and higher in children with idiopathic nephrotic syndrome.
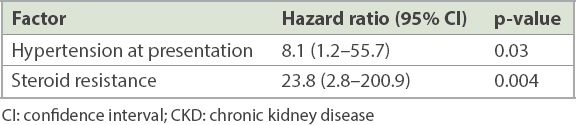
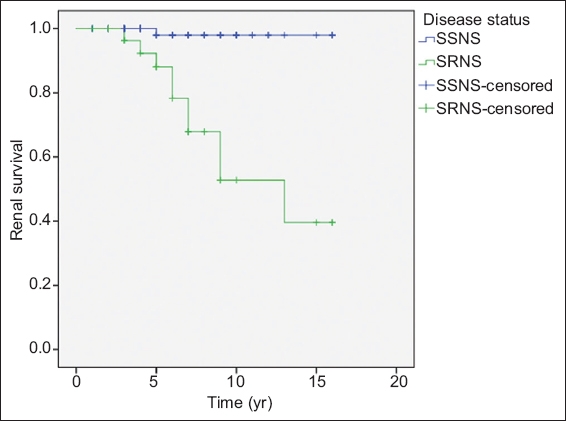
Fig. 2
Kaplan-Meier plot shows time to renal survival in children with steroid-sensitive nephrotic syndrome (SSNS) and steroid-resistant nephrotic syndrome (SRNS) (maximum follow-up time = 17 years).
DISCUSSION
In our cohort from Malaysia, we determined the renal survival time and predictive factors associated with progression to CKD among children with INS. Mendonça et al reported overall median renal survival time among Brazilian children with INS as 26 years.(2) The estimated median renal survival time for SRNS in our study was 13 years, which was comparable to the 14–15 years documented in previous studies.(2,10) The prevalence of CKD among children with INS in our study was 10.7%, which was also in line with previous studies that have reported CKD prevalence in the range of 3%–10%. However, studies from Taiwan, Turkey and Nigeria with larger sample sizes showed a lower prevalence rate (about 3%–4%) of CKD.(2,8,15-17)
Hypertension, AKI and microscopic haematuria were the features of SRNS,(16-18) as well as the predictive factors for progression to CKD.(2,8,11) However, in our cohort, those features did not differ between patients with SSNS and SRNS. The presence of hypertension was the only significant predictor of CKD. Hypertension in nephrotic patients has been attributed to sodium retention secondary to intrinsic factors, such as atrial natriuretic peptide resistance and upregulation of epithelial sodium channels, rather than as previously thought to be due to the activation of the renin angiotensin system.(19,20)
Yaseen et al reported that 50% of children with INS who developed AKI at presentation progressed to CKD.(21) Regardless of the underlying cause, the risk of progression to ESRD from initial AKI was 13–40 times higher due to continuous fibrotic changes in the renal interstitial and epithelial cells.(21) However, our patients showed a more favourable outcome. All six patients with AKI upon presentation did not require acute dialysis, and all patients were able to preserve normal renal function at the end of follow-up.
SRNS is a glomerular disease with unfavourable outcomes that poses a therapeutic challenge. A majority of patients in our cohort demonstrated complete remission after administration of second-line immunosuppression (cyclosporine or cyclophosphamide). These findings were in accordance with other cohorts from Thailand,(15) Japan(22) and Iran(23) when compared to less than 50% from Europe(10) and Turkey.(16) Banh et al have reported that INS among Asians has a better outcome when compared to the European populations.(24) This high response rate in Asian patients, when compared to Western data, was possibly due to the influence of ethnic and genetic differences. However, we were unable to proceed with genetic analysis for our cohort due to limited resources.
FSGS was a major determinant for poor renal outcomes in some studies.(8,15) However, we could not prove the significance of FSGS as an important predictive factor for progression to CKD among our children with INS. This could be attributed to the small sample size and limited number of renal biopsies done in our setting.
In many studies, advanced age at presentation was associated with increased incidence of FSGS and steroid resistance.(3,7,8,18) However, age was not found to be a significant predictor in our study. Most of our patients were aged less than ten years and this may explain the good response seen to second-line immunosuppression among our patients.
Poor understanding of illness by patients and parents, late presentation, limited resources and influence of traditional healers were challenges for the management of patients with chronic diseases,(25) including nephrotic syndrome. There were uncertain histological diagnoses in four patients with SRNS as a result of parental refusal of renal biopsy. Due to this limitation, treatment could not be optimised for them, and this may have possibly contributed to the progression of CKD in each of these patients.
The longest duration of our follow-up was 17 years. Perhaps a longer follow-up duration would allow us to assess for progression to ESRD. A larger scale, long-term multicentre prospective study is warranted in Southeast Asia to provide a better understanding of the progression of INS to CKD and ESRD in children.
In conclusion, the prevalence of CKD among our patients with INS and the median renal survival time were comparable to other studies. The presence of hypertension at presentation and steroid-resistant clinical course were significant predictors for developing CKD. Clinicians should thus remain alert to seek early specialist referral for diagnostic renal biopsy, so that patients at risk of progressive renal disease are managed more effectively and in a timely manner.


