Abstract
INTRODUCTION
Percutaneous transluminal angioplasty (PTA) is commonly used to treat patients with chronic limb-threatening ischaemia (CLTI). This study aimed to examine the mortality and functional outcomes of patients with CLTI who predominantly had diabetes mellitus in a multi-ethnic Asian population in Singapore.
METHODS
Patients with CLTI who underwent PTA between January 2015 and March 2017 at the Vascular Unit at Singapore General Hospital, Singapore, were studied. Primary outcome measures were 30-day unplanned readmission, two-year major lower extremity amputation (LEA), mortality rates, and ambulation status at one, six and 12 months.
RESULTS
A total of 221 procedures were performed on 207 patients, of whom 184 (88.9%) were diabetics. The one-, six- and 12-month mortality rate was 7.7%, 16.4% and 21.7%, respectively. The two-year LEA rate was 30.0%. At six and 12 months, only 96 (46.4%) and 93 (44.9%) patients were ambulant, respectively. Multivariate analysis revealed that preoperative ambulatory status, haemoglobin, Wound Ischaemia and foot Infection (WIfI) score, and end-stage renal failure (ESRF) were independent predictors of one-year ambulatory status. Predictors of mortality at one, six and 12 months were ESRF, preoperative albumin level, impaired functional status and employment status.
CONCLUSION
PTA for CLTI was associated with low one-year mortality and two-year LEA rates but did not significantly improve ambulation status. ESRF and hypoalbuminaemia were independent predictors of mortality. ESRF/CKD and WIfI score were independent predictors of loss of ambulation at six months and one year. We need better risk stratification for patients with CLTI to decide between initial revascularisation and an immediate LEA policy.
INTRODUCTION
Peripheral arterial disease (PAD) is a chronic atherosclerotic process that results in narrowing of the peripheral arterial vasculature, especially of the lower limbs.(1,2) The Trans-Atlantic Inter-Society (TASC II) guidelines reported that the total disease prevalence of PAD ranges from 3%–10%, increasing to 15%–20% in people aged over 70 years.(3) Chronic limb-threatening ischaemia (CLTI), the most severe manifestation of PAD, is defined by the presence of obstructive arterial disease associated with tissue loss that fails to heal within two weeks. The prevalence of CLTI is highest among diabetics, smokers and dialysis-dependent patients, and can result in limb loss or even death when left untreated. It is reported that about one-half of patients without revascularisation will either die or undergo major amputation within one year.(4)
Diabetes mellitus (DM) is one of the main risk factors for developing PAD. Patients with DM have a 3–4 times higher relative risk of developing PAD than non-DM patients.(5) In April 2016, the Ministry of Health, Singapore, declared a ‘war’ on DM in view of the fact that the local prevalence had increased from 8.2% in 2004 to 11.3% in 2010. In 2014, around 440,000 Singaporeans aged above 18 years had DM, and this number is projected to increase to 670,000 by 2030.(6) Given the increasing prevalence of DM, it is unsurprising that there is an increasing number of DM-related lower extremity amputations (LEAs) in Singapore.(7) Diabetics have a 10%–25% lifetime risk of developing foot ulcers(8) and 20 times increased risk of LEA.(9)
LEA has often been associated with a diminished quality of life and impaired functional status.(10,11) Moreover, patients with LEA require extensive resources and funds for rehabilitation and social care. In order to prevent amputation, restoration of pulsatile blood flow to the foot is imperative to aid wound healing.(12) As such, the past few decades have seen advances in the surgical treatment of threatened limbs, where emphasis is placed on a limb-salvage approach to pursue revascularisation through open bypass grafting or lower limb percutaneous transluminal angioplasty (PTA).(1) With these aggressive techniques, limb salvage rates of more than 80% at one year(1,13,14) have been reported. Data regarding the surgical outcomes of these revascularisation procedures has primarily focused on the primary patency and target limb revascularisation rates of the affected blood vessels. Although patency is important to assess the success of technical angioplasty, the main aim of the intervention is limb preservation and maintenance or improvement of functional status. Few studies have evaluated functional outcomes in the form of quality-of-life measures and ambulation status, but only in non-Asian populations.(15,16) The longer-term implications of limb revascularisation on quality-of-life and functional status, especially in an Asian population, remain unclear.
The aims of this study were to examine the functional outcome status, limb salvage and mortality rates of patients with CLTI up to two years after undergoing lower limb endovascular revascularisation at a tertiary vascular centre in Singapore.
METHODS
We conducted a retrospective review of 207 patients (221 interventions) with CLTI (Rutherford category 4 to 6) who underwent lower limb endovascular revascularisation with PTA from January 2015 to March 2017 at Singapore General Hospital, a tertiary vascular centre in central Singapore. All patients had at least one year of follow-up. Indications for PTA were rest pain or tissue loss. These patients were identified using the hospital’s diagnosis and operative code system. Their case notes and electronic records were reviewed. The local Centralised Institutional Review Board (CIRB no. 2018/2995) approved this study.
Premorbid variables collected included patient demographics, comorbidities, premorbid functional status, Rutherford classification, WIfI (Wound, Ischaemia and foot Infection) scores, preoperative toe pressure, and duration of wounds or symptoms. Outcomes measured included: one-, six- and 12-month mortality; length of stay in hospital; 30-day unplanned readmission; subsequent major LEA at two years; and six- and 12-month ambulatory status.
Arterial duplex ultrasonography of the index limb and toe pressure measurements were obtained prior to all interventions. All patients were seen by the cardiology and nephrology departments for optimisation of medications and risk profile prior to each intervention.
PTA was performed by one of the attending vascular surgeons in a fixed imaging hybrid operating room under either local, regional or general anaesthesia, depending on patient compliance, the complexity of the procedure and whether a concomitant ray amputation or wound debridement was also required. Intravenous antibiotics were administered at induction. Standard digital subtraction techniques were employed to maximise image quality while minimising the use of contrast agent. Carbon dioxide angiography was used in patients with contrast allergy or poor renal function. An ipsilateral antegrade common femoral approach was preferred for infrainguinal and infrapopliteal stenotic and/or occlusive lesions. A contralateral up-and-over approach was adopted only when severe disease of the ipsilateral common femoral artery precluded ipsilateral antegrade puncture, or when concomitant iliac lesions needed to be treated. Intra-arterial heparin (1,000–5,000 IU) was routinely administered via the intra-arterial sheath prior to intervention and only after a diagnostic angiogram had been performed. Intra- or transluminal crossing of the stenosis or occlusion was performed if possible. There was a low threshold to adopt a subintimal crossing technique when intraluminal crossing was unsuccessful, especially in the more calcified lesions. When antegrade lesion crossing failed, retrograde crossing from a distal puncture was attempted via the popliteal artery or the tibial vessels. Distal access was usually obtained using a micropuncture needle (MicropunctureÒ Access set, Cook Medical, Bloomington, IN, USA) under ultrasonography or fluoroscopic guidance. Femoropopliteal lesions were generally crossed using a 0.035-inch hydrophilic Terumo glidewire (Terumo Asia Holdings Pte Ltd, Singapore), and 0.018-inch or 0.014-inch hydrophilic guidewires (V18, PT2; Boston Scientific, Marlborough, MA, USA) were used for infrapopliteal PTA. Plain old balloon angioplasty was applied to both supra- and infrapopliteal lesions with bail-out stenting used selectively for flow-limiting and spiral after PTA dissections. Drug-eluting balloons and stents were used at the discretion of the interventionist. After angioplasty, all patients received at least single antiplatelet therapy.
All patients were evaluated in a specialist vascular outpatient clinic at one, three and six months after the procedure to assess wound healing and patient ambulatory progress. During the follow-up period, the following primary endpoints were captured: 30-day unplanned readmission; 30-day, six-month and one-year mortality; ambulation status at six and 12 months; and subsequent major amputation of the operated limb.
Major LEA was defined as any amputation above the ankle. The extent of the lower limb ischaemia was categorised using the Rutherford classification (Rutherford 4 = rest pain, Rutherford 5 = non-healing ulcer, Rutherford 6 = gangrene). The WIfI score was calculated based on three components: (a) wound (0 = no ulcer, no gangrene, 1 = small ulcer, no gangrene, 2 = deep ulcer or gangrene limited to toes, 3 = extensive ulcer or extensive gangrene); (b) ischaemia (0 = toe pressure > 60 mmHg, 1 = toe pressure 40–50 mmHg, 2 = toe pressure 30–39 mmHg, 3 = toe pressure < 30 mmHg); and (c) foot infection (0 = non-infected, 1 = mild with < 2 cm cellulitis, 2 = moderate, 3 = severe with systemic response or sepsis).(16)
Descriptive statistics of demographic and clinical variables were used based on patients or procedures as the unit of analysis, as appropriate. Survival probability was computed based on the individual, from the date of the first operation. Univariate analysis was conducted on categorical outcomes using logistic regression. Variables with p < 0.05 were selected for multivariate analysis. Among these variables, correlated variables were identified using a correlation coefficient > 0.4, and the variable with the lowest univariate p-value was chosen out of each group of correlated variables. The remaining variables were entered into a stepwise selection model based on Akaike’s information criterion (AIC). The AIC estimates the relative quality of a set of models based on fit and parsimony. Apart from 30-day unplanned readmission, which was analysed on a procedure level, other outcomes (mortality, amputation rate and ambulation status) were analysed on a patient level, with reference to the first procedure.
We also analysed the association between the demographic and clinical variables with survival on the patient level using the Cox proportional hazards model. Similarly, univariable analysis was conducted, and variables with p < 0.05 were entered into a stepwise multivariable Cox regression. All analyses were conducted with R version 3.4.2.(17)
RESULTS
A total of 228 limbs (221 procedures) in 207 patients (58.9% male, n = 122) received the intervention. The median follow-up period was 20.5 months (interquartile range [IQR] 15.3–27.8 months). Preoperatively, 146 (70.5%) of the 207 patients were ambulant. The demographics and clinical characteristics of the patients are shown in
Table I
Characteristics of patients who underwent lower limb angioplasty procedures (n = 207).
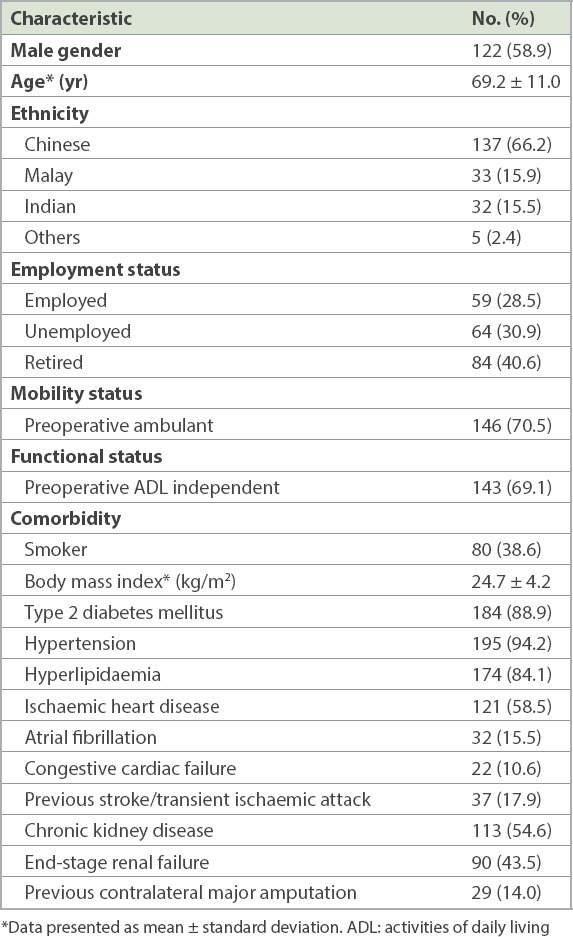
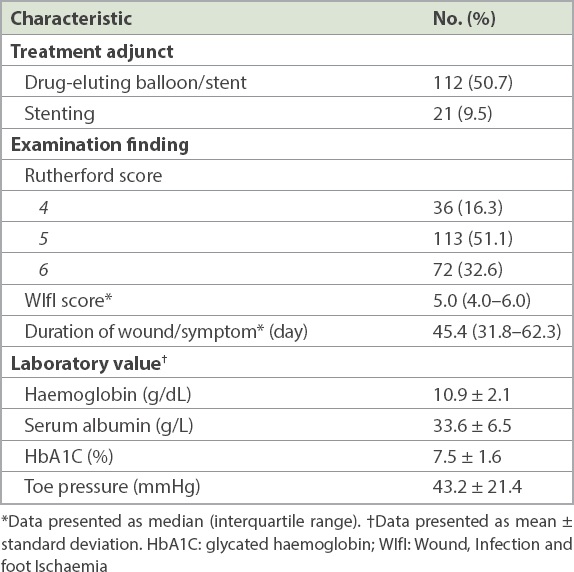
Table II
Pre-procedure characteristics (n = 221).
The overall survival of the 207 patients from their first procedure is shown in
Fig. 1
Chart shows the overall survival of patients undergoing lower limb angioplasty, with the shaded area representing the 95% confidence region.
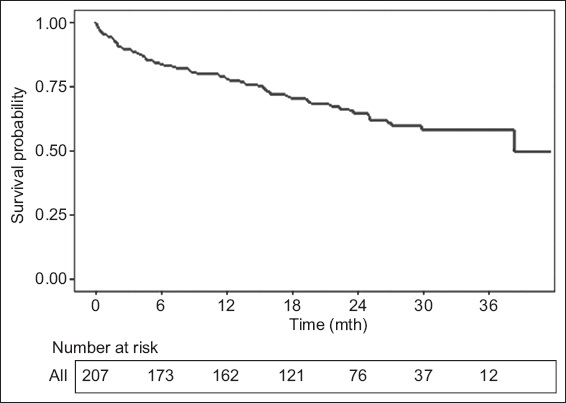
Table III
Multivariate analysis for post-procedure outcomes.
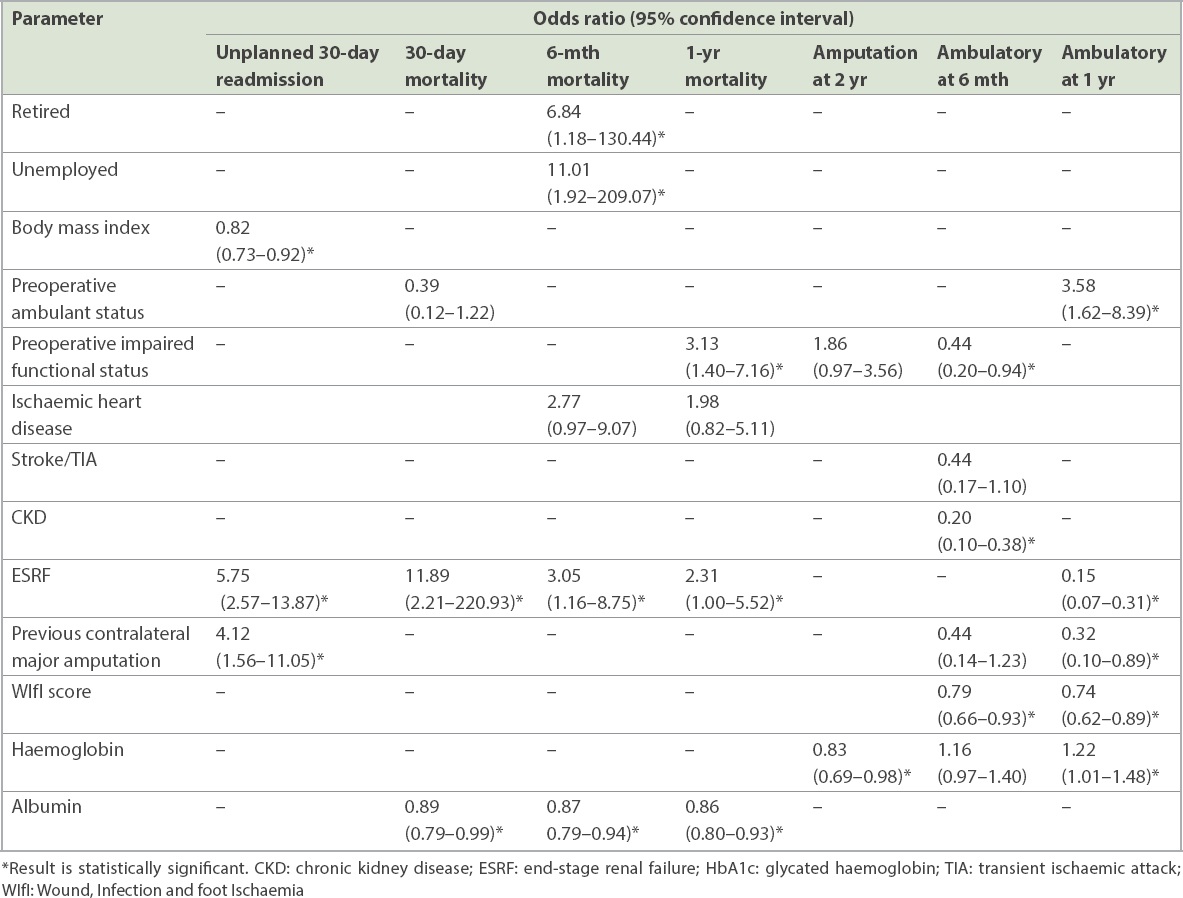
The 30-day unplanned readmission rate was 45 (20.4%) out of 221 procedures. Multivariate analysis showed that BMI (OR 0.82, 95% CI 0.73–0.92), ESRF (OR 5.75, 95% CI 2.57–13.87) and previous contralateral major amputation (OR 4.12, 95% CI 1.56–11.05) were independent predictors. At two years after the first procedure, 62 (30.0%) of the 207 patients had a major LEA. Multivariate analysis revealed that haemoglobin level (OR 0.83, 95% CI 0.69–0.98) was the only independent predictor of LEA.
Preoperatively, 146 (70.5%) of the 207 patients had independent ambulatory status. At six months and 12 months postoperatively, 96 (46.4%) and 93 (44.9%) patients had independent ambulatory status, respectively. Multivariate analysis showed that WIfI score (OR 0.79, 95% CI 0.66–0.93), CKD (OR 0.20, 95% CI 0.10–0.38) and preoperative impaired functional status (OR 0.44, 95% CI 0.20–0.94) were independent predictors of ambulation at six months. Out of 77 patients who were non-ambulant and surviving at six months, none had a WIfI score of 0, while 6 (7.8%) scored 1–3, 45 (58.4%) scored 4–6 and 26 (33.8%) scored 7–9. At one year, multivariate analysis showed that WIfI score (OR 0.74, 95% CI 0.62–0.89), ESRF (OR 0.15, 95% CI 0.07–0.31), preoperative ambulant status (OR 3.58, 95% CI 1.62–8.39), previous contralateral amputation status (OR 0.32, 95% CI 0.10–0.89) and haemoglobin level (OR 1.22, 95% CI 1.01–1.48) were independent predictors of ambulation status. Out of 67 patients who were non-ambulant and surviving at one year, 62 (92.5%) had a WIfI score of 4–9.
DISCUSSION
Despite a relatively low two-year LEA rate of 30.0%, less than half of the CLTI patients in our cohort could walk independently at six months and 12 months after lower limb revascularisation. The WIfI score and CKD/ESRF were independent predictors of ambulation at both time points. The 30-day mortality rate (7.7%) in the present study was higher than that in the literature and could be attributed to the fact that a large proportion of patients had cardiovascular risk factors and were dialysis dependent. If these patients could survive the perioperative period, the six-month (16.4%) and one-year (21.7%) mortality rates were in keeping with the literature. ESRF and low albumin level were independent predictors of mortality at all three time points. Interestingly, the presence of DM was not predictive of any outcome.
The 30-day mortality rate was also higher than that of other cohort studies, which have reported rates of about 2%–6%.(18-22) This may be attributable to our institution’s low threshold for endovascular treatment in patients with CLTI regardless of the premorbid status and patient/family preferences to not have LEA. Surgically fitter patients may have also undergone a bypass procedure instead of endovascular treatment. Our high proportion of patients with ESRF also contributed to the higher 30-day mortality rate. Patients with ESRF have multiple systemic comorbidities and their arteries are affected by severe calcification and multiple distal occlusions.(23,24) Vogel et al(22) and Meyer et al(25) found that patients with ESRF who undergo endovascular treatment for CLTI have an increased risk of amputation or death compared to those with normal renal function. Biancari et al(26) evaluated 1,425 patients who underwent infrainguinal revascularisation and found that patients with ESRF had significantly lower overall survival at three years (27.1% vs. 59.7%), limb salvage (57.7% vs. 83.0%) and amputation-free survival (16.2% vs. 52.9%) compared to patients with normal or impaired renal function.
The effect of preoperative malnutrition on outcomes in patients undergoing major vascular surgery is unclear. In this study, low albumin contributed to higher mortality rates at all three time points. A 2016 study(27) of 15,002 patients who underwent open abdominal aortic aneurysm repair and endovascular abdominal aortic aneurysm repair showed that preoperative hypoalbuminaemia is associated with increased postoperative morbidity and mortality. Another study(28) of 5,110 lower extremity bypass procedures showed that low albumin was independently associated with increased mortality (OR 1.8, 95% CI 1.3–2.6). Other studies within the vascular surgery literature have identified hypoalbuminaemia as a predictor of poor outcomes. Albumin was shown to be a protective factor against major adverse events, including stroke, death and cardiac events, in a study on nutritional status and carotid endarterectomy.(29) In another study, hypoalbuminaemia was shown to be independently associated with mortality after non-traumatic major LEA.(30) Hypoalbuminaemia, an indication of poor nutritional status, is most likely reflective of overall poor health and potential susceptibility to infectious complications.(31)
Although anatomical patency outcome and freedom from restenosis and reintervention are measures that demonstrate technical and device success, they do not reflect the ultimate goal of interventions for CLTI, which is preservation of limb with ambulation without high morbidity or mortality. 70.0% of our cohort still had the qualifying limb after two years, similar to other studies that found that survivors are likely to retain their limb over time.(32,33) However, the ambulatory outcomes in our study were comparatively poor, possibly owing to poor patient motivation and underdiagnosed depression.
Our study showed that WIfI score and CKD/ESRF contributed to loss of ambulation at both six months and one year. This is consistent with the current literature. Darling et al(34) evaluated 596 limbs with CLTI undergoing endovascular interventions and concluded that WIfI predicted one-year amputation, restenosis events and wound healing.
Several studies in non-Asian populations have shown that a successful angioplasty may not contribute to an improved functional outcome. Davies and El-Sayed(18) evaluated 728 patients who underwent lower limb angioplasty and found that less than 40% of patients maintained ambulation after one year. In a smaller study, Duggan et al(35) found no significant difference in the quality of life between patients who had undergone a successful limb salvage procedure and those who had not.
To our knowledge, no study has investigated post-angioplasty functional outcomes in an Asian population. Our finding that less than half of the patients could walk independently at six and 12 months after lower limb revascularisation is important in our local context, where much emphasis is placed on mobility. The loss of mobility is perceived as a major disability and represents lack of relevance to the workforce in our society.
In this case series, low haemoglobin level was the only independent predictor of subsequent LEA. This is consistent with findings from a study(36) on 925 patients with PAD, which showed that a lower level of haemoglobin is associated with a higher risk of mortality and amputation (hazard ratio 1.20, 95% CI 1.09–1.32). Chronic anaemia increases left ventricular preload, reduces afterload and causes increased cardiac output.(37) Over time, this results in left ventricular hypertrophy, a well-recognised risk factor for PAD and all-cause mortality.(38,39) Similarly, anaemia has been shown to be a risk factor for adverse outcomes in CKD.(40) Dunkelgrun et al(41) studied the contribution of anaemia to the risk of perioperative and long-term cardiac outcome in 1,211 patients undergoing elective non-cardiac open vascular surgery. They found that the presence and severity of anaemia are significant predictors of 30-day and five-year cardiac events, regardless of underlying heart failure or renal dysfunction. The importance of anaemia as a risk factor for adverse outcomes in surgery has been well-established, but ours is one of the few studies that report on the relationship between anaemia and risk of LEA. Whether correcting anaemia (e.g. blood transfusion) could improve the limb and general prognosis of patients with PAD requires further investigation.
The presence of DM was not predictive of any outcomes. This is a surprising finding given that DM is widely known to contribute to greater complexity of vascular disease.(42,43) DM is characterised by hyperglycaemia, dyslipidaemia and insulin resistance,(44,45) which lead to derangements in vessel wall, fostering development and progression of PAD.(46) A possible reason is that the glycated haemoglobin control of the patients in this study was relatively good (median 7.2%) at the time of PTA.
Given the relatively high 30-day mortality rate and low ambulatory rate in this study, we may be overtreating some patients with CLTI. A risk stratification model for patients with CLTI consisting of both patient and wound factors may better guide management instead of subjecting the majority to an angioplasty-first approach. The relatively higher 30-day mortality rate shows that patient factors such as ESRF, low albumin and preoperative employment status may negate the benefits of surgical intervention. For patients who survived beyond 30 days, wound factors such as WIfI score and patient factors such as ESRF, stroke/TIA and preoperative ambulatory status predicted improvement in functional performance.
Currently, various scoring systems are used in the context of CLTI that seek to risk stratify patients and determine management. The TransAtlantic Inter-Society Consensus (TASC) is a widely available classification used to grade the severity of PAD.(47) However, it contributes little to the management of CLTI, because the majority of infrapopliteal lesions are classified as TASC D. The Wagner and the University of Texas(5) wound scores have been reported to accurately predict the outcomes of diabetic foot ulcers.(48,49) However, healing in CLTI can change profoundly depending on the outcome of interventions, and these classifications are unable to predict outcomes after intervention, as they do not include procedure or patient characteristics. As the healing potential of CLTI limbs is associated with both patient as well as wound characteristics, a risk stratification model comprising both wound factors as well as patient factors may be useful in deciding the best and most cost-effective approach for our patients with CLTI.
In particular, the role of frailty in determining the outcomes of vascular surgery is being increasingly recognised. Frailty is defined as a clinical syndrome secondary to the decline of physical activity level or cognitive function that leads to adverse outcomes.(50) Previous reports have shown the influence of frailty on revascularisation outcomes in patients with PAD.(51,52) In these studies, a modified frailty index was used as an indicator of the presence of frailty. Several factors in our study are associated with frailty. It would be interesting in a prospective study to include other objective measures of frailty, including grip strength and weight loss.
The limitations of this study include its retrospective design with the associated selection and information biases. In addition, we did not obtain information regarding the technical success and patency rates of the interventions, and assumed that all interventions reported were technically successful. Information on the anatomical location of the diseased vessels was also not collected. Another shortcoming of this study is the method chosen to measure the functional outcomes and quality of life of the patients. Reports from Europe have suggested that disease-specific quality-of-life questionnaires designed for patients with PAD may provide more reliable quality-of-life data.(53,54) This should be included as a measure in any future prospective study on CLTI outcomes.
In conclusion, the mortality and two-year lower extremity amputation rates following lower endovascular revascularisation for CLTI are low and in keeping with those in the current literature. However, lower limb angioplasty did not significantly improve the ambulation status or functional outcomes in half of our patients. ESRF and low albumin levels were independent predictors of mortality at all three time points. Anaemia was an independent predictor for LEA, while CKD/ESRF, preoperative ambulatory/functional status and WIfI score were independent predictors of loss of ambulatory status at six months and one year. These factors can potentially be utilised to risk stratify our patients with CLTI to decide who might benefit more from a limb-salvage strategy, instead of subjecting all of these high-risk patients to a revascularisation-first approach. This study also opens a new field for investigation into whether correction of anaemia and albumin preoperatively may improve post-surgical outcomes in Asian patients with CLTI.
SUPPLEMENTARY MATERIAL
About the Senior Author

Dr Tang Tjun Yip graduated from Queens' College in Cambridge, United Kingdom, and qualified from Addenbrooke's Hospital, Cambridge, in 2000 with a Distinction in Surgery. He was awarded his Doctorate of Medicine by the University of Cambridge in 2009 for research into carotid plaque inflammation. Dr Tang is currently a Senior Consultant in vascular and endovascular surgery at Singapore General Hospital, with active subspecialty interests in diabetic foot salvage, endovenous surgery and deep vein stenting. He has more than 290 peer-reviewed publications. Recently accorded the National Medical Research Council Clinician Scientist Award, he is the first vascular surgeon in Singapore to be bestowed this prestigious career award. He is a Fellow of both the Royal College of Surgeons of England and the Royal College of Physicians and Surgeons of Glasgow.


