Dear Sir,
We report a rare case of recurrent pituitary macroadenoma encasing and invading the right optic track. While bowing of the optic chiasm is common, invasion and encasement of the optic track has, to the best of our knowledge, never been reported.
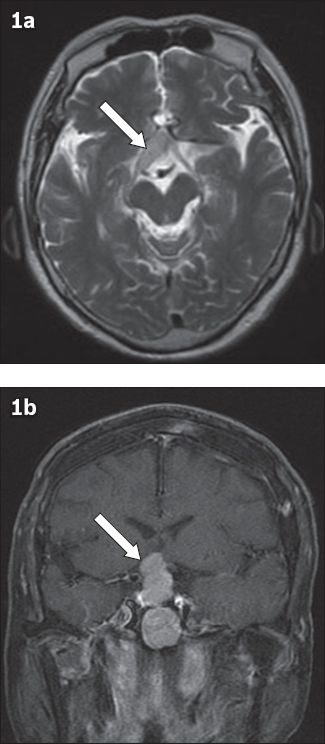
A 75-year-old Chinese woman underwent transsphenoidal surgery in June 1999 for pituitary macroadenoma. Postoperative hormonal analysis revealed no anterior pituitary hormonal deficiency. However, the patient had polyuria; desmopressin was started but subsequently stopped due to noncompliance. Follow-up magnetic resonance (MR) imaging in December 1999 and December 2000 revealed recurrence of the pituitary tumour, which was gradually increasing in size. Subsequent Gamma Knife radiosurgery in February 2001 resulted in a transient reduction in lesion size. However, further scans in February 2003 showed an increase in size of the pituitary tumour. The patient refused open surgery and instead opted for radiotherapy. Subsequent clinic follow-ups till June 2003 were uneventful and she defaulted on follow-up thereafter. The patient presented again in July 2011 with complaints of fever, dysuria, increased frequency of urination, and loss of both temporal and right inferior nasal visual fields. MR imaging (
Fig. 1
MR images show (a) pituitary macroadenoma encasing and invading the right optic track (arrows) and (b) extension into the right cavernous sinus.
Pituitary adenomas constitute 10%–15% of all brain tumours and usually manifest with characteristic endocrine disturbances related to either hypopituitarism or hypersecretion of certain hormones such as growth hormone, prolactin, adrenocorticotropic hormone and thyroid-stimulating hormone.(1) Hardy and Vezina classified pituitary tumours according to their suprasellar extension,(2) as Type A: the suprasellar expansion bulges into the chiasmatic cisterns, but does not reach the floor of the anterior third ventricle; Type B: the tumour reaches the floor of the third ventricle; Type C: a voluminous suprasellar expansion bulges largely into the third ventricle up to the foramen of Monro; and Type D: rare aberrant expansions in temporal or frontal fossa.(2) Wilson modified Hardy’s classification to include an extension of the pituitary tumour into or beneath the cavernous sinus as Stage E.(3) Knosp et al further classified parasellar growth or extension into the cavernous sinus into five stages (i.e. Stages 0–4).(4)
The transsphenoidal approach is generally favoured over transcranial surgery for excision of most pituitary adenomas, as the former causes less injury to the pituitary gland and allows rapid recovery of pituitary endocrinological function and visual impairment. However, controversy still exists over the surgical approach for adenomas with suprasellar extensions. Regular careful observation is recommended after surgery, with hormonal replacement when needed. Radiotherapy is reserved for controlling a residual tumour that is surgically unresectable, especially when cavernous sinus invasion is present and for the prevention of late recurrences.(5)
Yours sincerely,


