Abstract
A 56-year-old Chinese man presented with giddiness and vertigo. Subsequent chest radiography showed the classic scimitar sign of an abnormal pulmonary venous return. Further evaluation with non-contrast computed tomography substantiated the finding of a partial anomalous venous drainage pattern and identified an associated rare lung anomaly, horseshoe lung. The imaging findings of scimitar syndrome and its association with horseshoe lung are reviewed.
CASE PRESENTATION
A 56-year-old Chinese man had chest radiography performed as part of a routine work-up for a recent onset of vestibular neuronitis. He presented with severe giddiness and positional vertigo. There was no history of cough, fever or breathlessness. The clinical examination revealed no rales, crepitation or wheezing. There was no previous history of chest infection. The pulse rate, blood pressure and saturation of peripheral oxygen (SpO2) were normal. What do the frontal chest radiograph (
Fig. 1
Posteroanterior radiograph of the chest.
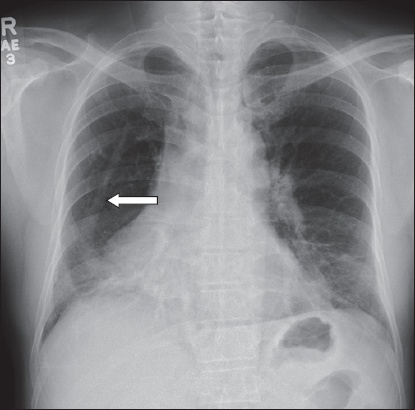
Fig. 2
Axial CT images of the mid and lower thorax on (a & b) soft tissue and (c) lung windows.
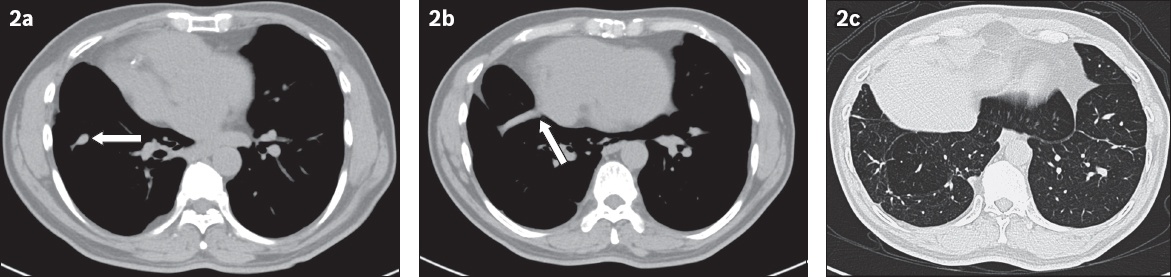
IMAGE INTERPRETATION
The chest radiograph shows a vertical curvilinear opacity parallel to the right heart border, which gradually increases in width along its caudal course before merging with the right lower cardiomediastinal silhouette, producing the scimitar sign (arrow,
Fig. 3
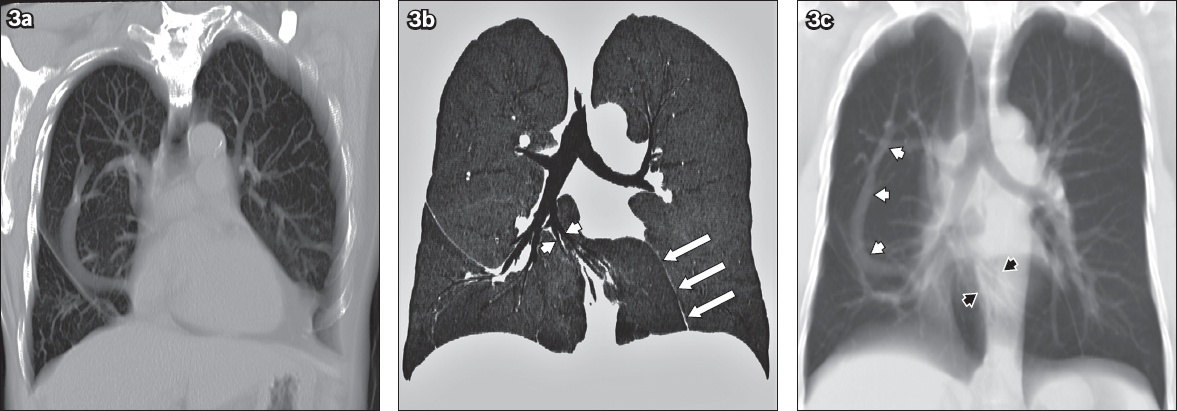
Anatomical configuration of a scimitar vein and horseshoe lung. (a) Reconstructed oblique coronal CT image shows partial anomalous venous return in the entire extent and drainage into the supradiaphragmatic inferior vena cava. (b) Coronal CT minimal intensity projection image shows the isthmus of horseshoe lung, altered course of the right middle lobe segmental bronchi (white arrowheads) and pleural line of fusion (white arrows). (c) Thick reconstructed coronal CT image shows the scimitar vein in its entirety (white arrowheads) and altered course of the right middle lobe bronchovascular bundle (black arrowheads).
DIAGNOSIS
Scimitar syndrome with horseshoe lung.
CLINICAL COURSE
The patient responded to treatment for the presenting symptoms of giddiness and positional vertigo. No further assessment of scimitar syndrome or associated cardiac anomaly was pursued.
DISCUSSION
Scimitar syndrome is characterised by an anomalous pulmonary venous return, in which the venous channel from the lung drains into the systemic vein rather than the left atrium. It falls under the broad category of congenital pulmonary venolobar syndrome.(1) This complex pulmonary malformation has an incidence rate of 1–3 per 100,000 and a 2:1 female preponderance. The anomalous pulmonary vein typically drains the lung partially into the systemic circulation, which is usually the infradiaphragmatic inferior vena cava. Other rare drainage sites are the right atrium, superior vena cava, portal vein, hepatic vein and azygous vein.(2) This drainage pattern leads to left-to-right shunting which, if significant, can result in the development of pulmonary arterial hypertension. Although the anomaly is usually right-sided, left-sided scimitar syndrome(3) and cases of bilateral scimitar syndrome(4) have been reported. Two forms have been described based on the age of presentation: infantile and adult forms. The infantile form is more symptomatic and commonly associated with congenital cardiac defects such as atrial septal defect, persistent left superior vena cava and vascular rings. The adult form is less severe, mostly asymptomatic and has a relatively good prognosis.
Clinically, the scimitar anomaly can be asymptomatic and detected incidentally on imaging, or present with severe pulmonary hypertension (typically in the infantile form with associated congenital cardiac defects). Radiographically, a clue to the presence of an abnormal pulmonary venous return is the demonstration of a curvilinear opacity in the lung that starts from the hilum, has a parallel course to the heart border and widens inferiorly toward the diaphragm, such that it resembles a scimitar (i.e. Turkish sword) (
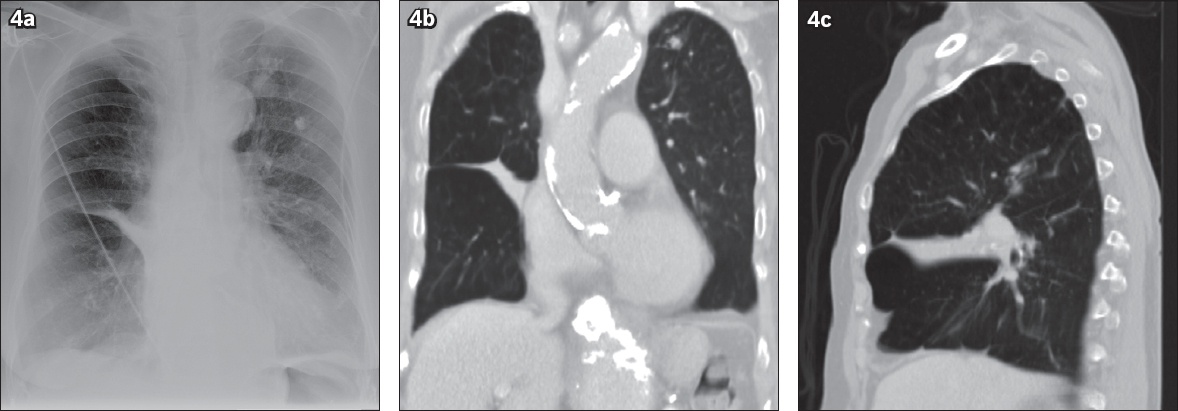
The scimitar sign, which was once thought to be diagnostic of scimitar syndrome, can be mimicked by other benign vascular anomalies such as a meandering pulmonary vein and an anomalous single unilateral pulmonary vein.(5) A meandering pulmonary vein occurs when one of the pulmonary veins draining into the left atrium has an anomalous pulmonary course. Another mimic of the scimitar sign is an anomalous single unilateral pulmonary vein, which is a single pulmonary trunk draining all the bronchopulmonary segments to the left atrium. It is clinically important to distinguish these benign entities from scimitar syndrome to avoid unnecessary intervention and imaging. As middle lobe collapse, pulmonary sequestration, pulmonary venous varix and intrapulmonary collaterals secondary to obstruction of the main pulmonary vein can also mimic the scimitar sign, this imaging sign may be suggestive but is not diagnostic of scimitar syndrome.(6) The radiographic opacity of middle lobe collapse often has a more horizontal orientation compared to the scimitar vein, which courses in a vertical and curvilinear fashion (
Fig. 4
Imaging mimics of the scimitar sign. (a) Posteroanterior chest radiograph shows oblique tubular opacity in the right middle zone, which merges with the right heart border and resembles the scimitar sign. (b & c) Coronal and sagittal minimal intensity projection CT images show triangular opacity in the right middle zone due to right middle lobe collapse.
These findings can be confirmed by lateral chest radiography or CT (Figs.
The diagnostic value of angiography is limited in cases of pulmonary atresia or high-grade stenosis; in such cases, MR imaging and CT are helpful alternatives to evaluate extracardiac venous and arterial anatomy.(7) Preoperative imaging of scimitar syndrome should aim to evaluate: (a) whether the pulmonary anomalous return is total or partial; (b) presence of pulmonary hypertension; (c) left-to-right shunt fraction; (d) presence of associated congenital cardiac defects; (e) presence of aortopulmonary collaterals; and (f) presence of anomalous arterial supply to the hypoplastic lung. Surgical repair is warranted if the shunt fraction (Qp:Qs) is > 2. The surgical options of direct reimplantation of the scimitar vein and intracardiac baffle repair are generally safe and efficient. However, higher mortality and complication rates are observed in infants and patients with associated pulmonary arterial hypertension.(8)
Horseshoe lung, a term that was first used by Spencer in 1962, is a rare lung malformation occurring in almost 80% of patients with scimitar syndrome.(9,10) In this rare lung anomaly, the posterobasal segments of both lungs usually show fusion in the midline through an isthmus of normal lung tissue. A parietal pleural defect allows for direct or indirect adherence of lung parenchyma through the intervening visceral pleura.(11) The isthmus of normal lung tissue lies posterior to the pericardial sac but anterior to both the oesophagus and aorta. Unilateral pulmonary hypoplasia is a constant feature of horseshoe lung and the bronchovascular supply to the isthmus is usually from the hypoplastic lung. Frank et al described the radiographic signs of horseshoe lung on chest radiographs; these included the presence of a well-defined area similar to an inferior accessory lobe and a fissure medial to the left lung.(9) This fissure corresponds to a pleural line separating the displaced right lung and the adjoining left lung, and is a consistent finding of horseshoe lung on CT.(12) The fused posterobasal segments of the right and left lungs may show imaging features of air trapping, suggesting possible stenosis of anomalous bronchus of the isthmus.(13) The functional importance of excluding a stenosed anomalous bronchus lies in the fact that impaired bronchial clearance results in recurrent pneumonia in the horseshoe lung segment, necessitating pneumonectomy or lobectomy. In the absence of other malformations, the prognosis of scimitar syndrome with horseshoe lung primarily depends on the pulmonary arterial pressure. A large shunt fraction is associated with a high risk of developing pulmonary arterial hypertension, although the patient is usually symptomatic in infancy or childhood. In cases of mild to absent left-to-right shunt, the patient is usually asymptomatic due to near-normal pulmonary arterial pressure and the absence of pulmonary hypertension. Hence, it can be deduced that an isolated association with horseshoe lung has little bearing on the prognosis of scimitar syndrome.(12)
In conclusion, scimitar syndrome and horseshoe lung have characteristic imaging features. Up to 80% of reported cases of horseshoe lung have been seen in association with scimitar syndrome. Therefore, it is essential for the radiologist to be aware of this rare entity when evaluating a patient with scimitar syndrome. Multiplanar cross-sectional imaging studies aid in the assessment of underlying anatomy and differentiation among various imaging mimics.
SMJ-58-33.pdf


