Abstract
A 92-year-old woman presented to the emergency department with urinary symptoms, fever and suprapubic tenderness. Her inflammatory markers were raised. Urine and blood cultures were negative. Computed tomography performed to look for a source of sepsis showed distension of the uterine cavity with high-attenuation fluid, an air-fluid level and gas locules along the uterine wall. The causes, clinical presentation and imaging features of pyometra are discussed.
CASE PRESENTATION
A 92-year-old Chinese woman with a history of hypertension, diabetes mellitus and ischaemic heart disease presented to the emergency department with constipation, urinary incontinence and reduced oral intake of five days’ duration. On examination, she was febrile (38.2°C) with suprapubic tenderness. Renal punch was negative. Initial laboratory markers revealed leucocytosis of 27.2 × 109/L (normal 4.0–9.6 × 109/L), C-reactive protein of 252.3 mg/L (normal 0.0–5.0 mg/L), serum glucose level of 15.0 mmol/L (normal 0.0–11.1 mmol/L) and glycated haemoglobin of 8.7% (normal < 6.0%). Her urine and blood cultures were negative for bacteria, but urine phase-contrast microscopy revealed leucocytes in her urine. Computed tomography (CT) of her abdomen and pelvis was performed two days into her admission (
Fig. 1
(a) Sagittal and (b) axial contrast-enhanced CT images of the abdomen and pelvis.
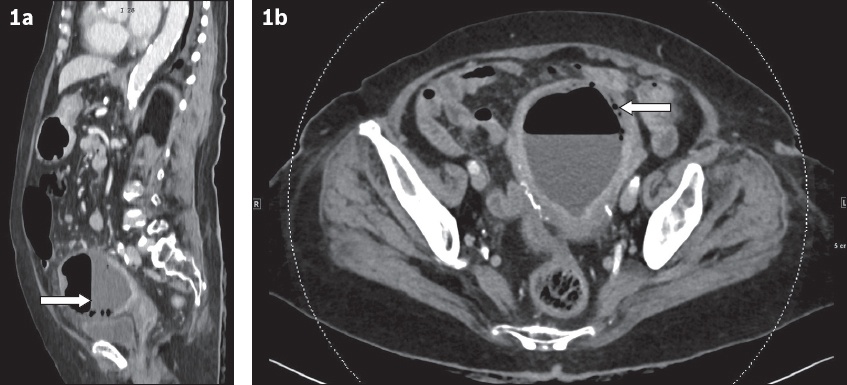
Fig. 2
(a) Transverse and (b) sagittal transvaginal US images.
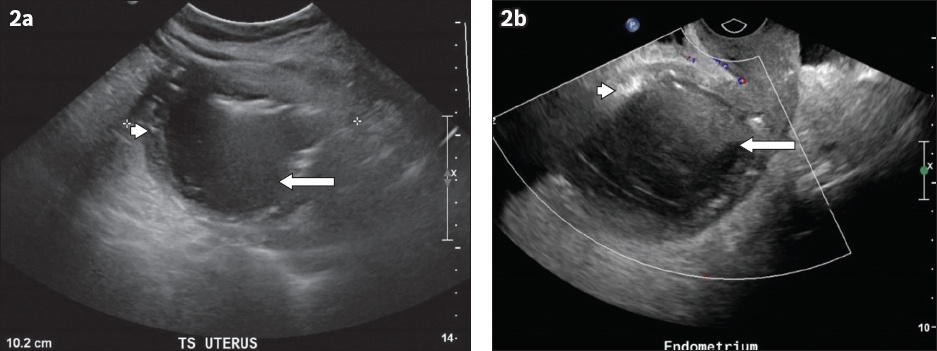
IMAGE INTERPRETATION
Sagittal contrast-enhanced CT image in the portovenous phase shows distension of the endometrial cavity with high-attenuation fluid and an air-fluid level (arrow,
DIAGNOSIS
Pyometra.
CLINICAL COURSE
The patient was initially treated for urosepsis with a combination of intravenous amoxicillin/clavulanate and gentamicin. Upon the imaging diagnosis of pyometra, she was reviewed by the gynaecology department and her antibiotic regimen was altered to ceftriaxone and metronidazole. An endometrial pipelle was used to drain the pus from the endometrial cavity, with no cervical dilation performed. A Pap smear of her cervix showed no malignant cells. Culture of the pus grew Peptostreptococcus (Gram-positive anaerobe, sensitive to metronidazole) and Escherichia coli (Gram-negative, sensitive to ceftriaxone and gentamicin). Her suprapubic pain and urinary symptoms continued to improve, while her inflammatory markers were on a downward trend for the remainder of her stay. She was discharged well on Day 7 of her admission.
DISCUSSION
Pyometra refers to the accumulation of pus in the endometrial cavity as a result of infection, and is thought to represent a chronic form of endometritis. It is a rare condition with a reported incidence of between 0.1% and 0.3% of all gynaecological attendances.(1,2) It mainly occurs in the postmenopausal age group and has a reported incidence of up to 13.6% among such patients.(3) Patients typically present with suprapubic pain, fever, chills, postmenopausal bleed and, on occasion, purulent vaginal discharge.(4)
For pyometra to occur, there must be interference with the natural drainage of the uterine cavity by causes such as uterine malignancy, pelvic inflammatory disease, prior irradiation, cervical stenosis or imperforate hymen in younger patients. Its occurrence in the elderly age group should always raise suspicion for malignancy,(3-7) such as vaginal squamous cell carcinoma or cervical carcinoma. Our patient’s Pap smear result showed no malignant cells. However, she had poorly controlled diabetes mellitus. While its association with pyometra was not well established in our literature review, we noted with interest the presence of poorly controlled diabetes mellitus in several prior reports of pyometra.(3,8) A possible association may well be explored in a larger case series.
CT and US are useful for image characterisation in pyometra. On CT, there is typically distension of the endometrial cavity with complex fluid, inflammatory fat stranding in the parametrium and free fluid in the pouch of Douglas. Gas bubbles or an air-fluid level may also be seen, as in the case of our patient. On US, there is complex fluid with internal echoes and debris, increased vascularity on colour Doppler imaging reflecting hyperaemia, and echogenic foci representing gas. Typically, there is also exquisite tenderness to any slight uterine movement during the sonographic or physical examination.(9-11) On magnetic resonance imaging, the fluid within the endometrial cavity is hyperintense on T2-weighted imaging and shows restricted diffusion; discontinuity of the uterine wall may also be evident.(12)
Although the diagnosis is typically straightforward given the characteristic imaging appearances, particularly on CT, one radiological mimic to be aware of is necrosis of a uterine fibroid. With causes such as torsion of a pedunculated fibroid or rapid overgrowth of the fibroid’s vascular supply, necrosis of uterine fibroids used to be uncommon. However, it has become more common recently due to the proliferation of uterine artery embolisation techniques, and careful history-taking with the patient is required.(13)
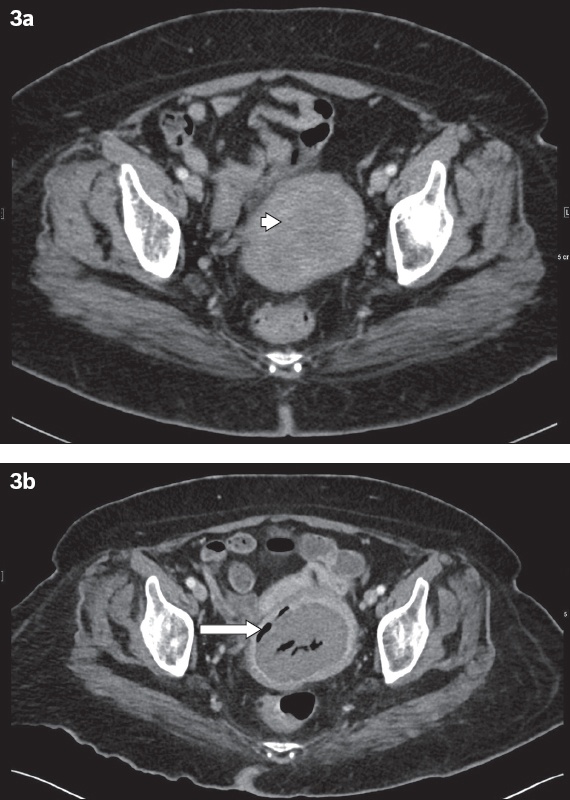
We herein report the case of a patient with a known intramural fibroid that subsequently underwent necrosis, mimicking pyometra on CT (
Fig. 3
Necrosis of a uterine fibroid in a 53-year-old woman. Transverse contrast-enhanced CT image shows (a) a hypodense, circumscribed fibroid at the left uterine wall (arrowhead); and (b) gas locules that appeared three months later in the centre of the fibroid and at its circumference (arrow), which is consistent with necrosis. No perilesional fat stranding is noted.
The mainstay of treatment for pyometra is antibiotics and drainage. Although the purulent material can typically be drained conventionally under anaesthesia via cervical dilatation and drainage, in our case, the pus was drained with only the use of an endometrial pipelle, as no obstructing cervical mass was present. In instances where these methods are not possible, US-guided percutaneous drainage has been described in the literature.(14)
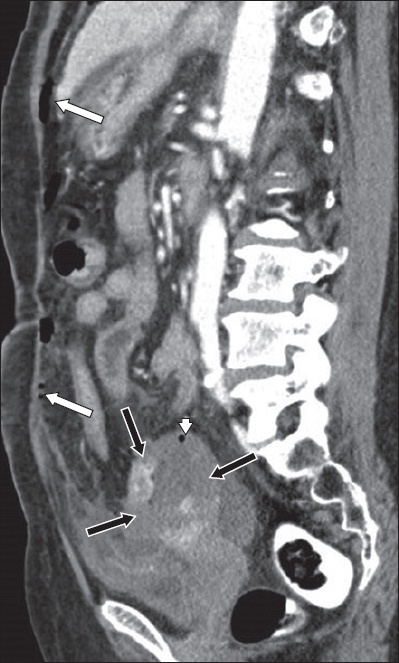
Although rare and mostly described in case reports, spontaneous perforation of pyometra is a feared complication that may result in diffuse peritonitis and pneumoperitoneum (
Fig. 4
Spontaneous perforation of pyometra in a 63-year-old woman. Sagittal contrast-enhanced CT image shows free intraperitoneal gas (white arrows), discontinuity of the uterine walls (black arrows) and an intramural gas locule in the uterine wall (arrowhead).
In summary, this case illustrates the characteristic imaging findings in a case of pyometra. The imaging diagnosis of this condition should be made in tandem with corroborative clinical and laboratory findings as well as sonographic examination, as the CT imaging features potentially overlap with those of uterine fibroid necrosis. Perforated pyometra is a dreaded complication that can be excluded with CT imaging.
SMJ-60-490.pdf


