Abstract
Ebstein’s anomaly is a congenital malformation characterised by tricuspid valve pathology with right heart enlargement. Cases of Ebstein’s anomaly can vary widely in severity, anatomy and presentation. In this article, we presented three cases of Ebstein’s anomaly and discussed the presentation as well as electrocardiographic (ECG) changes. Patients may first present to their primary care physicians with cardiac symptoms such as reduced effort tolerance together with an abnormal ECG. ECG changes suggestive of right heart enlargement are important in the initial consideration and eventual formal diagnosis of the condition.
CASE 1
CLINICAL PRESENTATION
A 26-year-old man with no past medical history presented with central chest discomfort of two weeks’ duration. This was associated with reduced effort tolerance. The patient denied any palpitations, syncope, lower limb swelling or any family history of cardiac problems. On physical examination, he had a blood pressure of 120/80 mmHg and heart rate of 80 beats per minute (bpm). Auscultation of the precordium revealed a Grade 3 pan-systolic murmur that was loudest over the lower left sternal edge without radiation. The first and second heart sounds were normal. Lung sounds were clear and there was no pedal oedema. What does the electrocardiogram (ECG) in
Fig. 1
ECG shows normal sinus rhythm with right bundle branch block.
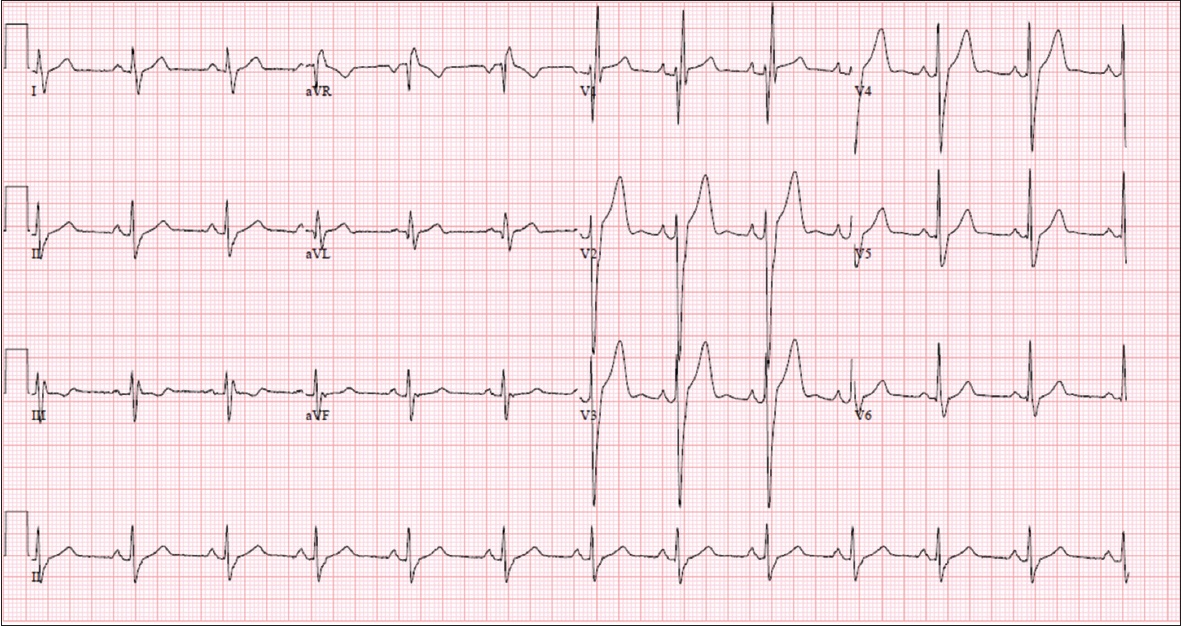
ECG INTERPRETATION
The ECG in
CLINICAL COURSE
Transthoracic echocardiography was performed. It showed a sail-like tricuspid valve with apical displacement of the septal leaflet and atrialisation of the right ventricle (RV) with dilated right atrium (RA) and RV. There was trivial tricuspid regurgitation (TR). Left and right ventricular systolic function was preserved. The patient remained asymptomatic, and cardiac magnetic resonance (MR) imaging was scheduled to further assess the anomaly.
CASE 2
CLINICAL PRESENTATION
A 14-year-old boy with a history of Ebstein’s anomaly was scheduled for a follow-up visit. He was otherwise well and denied any exertional symptoms. There was no chest pain, syncope, palpitations, orthopnoea or pedal oedema. The patient’s blood pressure was 100/80 mmHg and heart rate was 65 bpm. Cardiac examination showed a 2/6 pan-systolic murmur over the lower left sternal edge. ECG was performed (
Fig. 2
ECG shows normal sinus rhythm and QRS fragmentation.
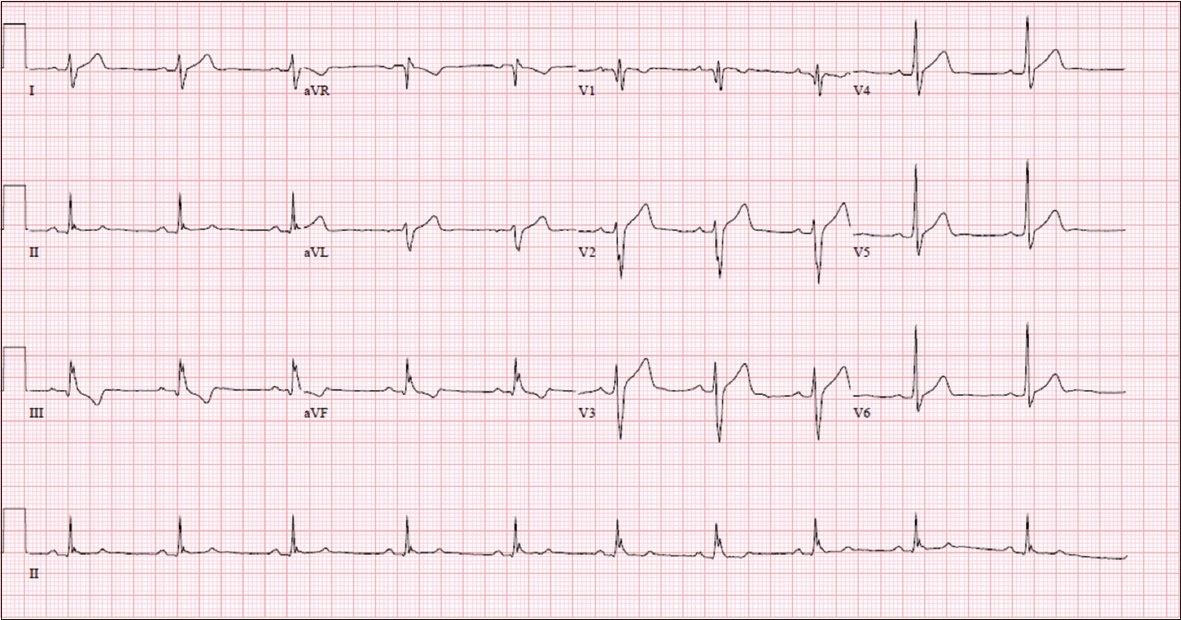
ECG INTERPRETATION
The ECG in
CLINICAL COURSE
Transthoracic echocardiogram showed significant apical displacement of the septal tricuspid leaflet with an enlarged RA and RV suggestive of Ebstein’s anomaly (
Fig. 3
Echocardiogram in apical four-chamber view shows significant apical displacement of the septal tricuspid leaflet with large RA and right ventricle. Note the sail-like appearance of the anterior tricuspid leaflet. LA: left atrium; LV: left ventricle; RA: right atrium
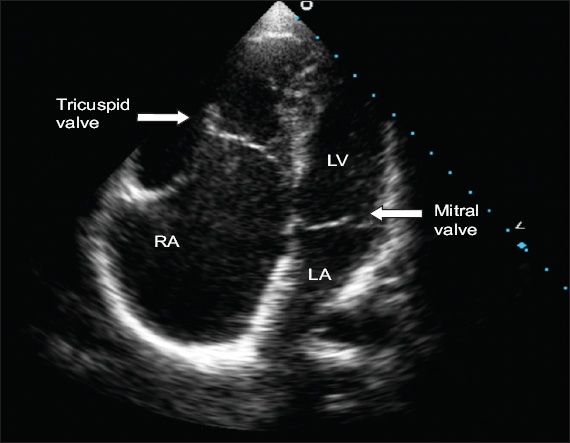
Fig. 4
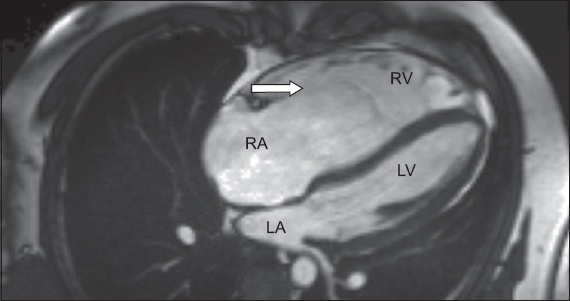
Cardiac MR imaging in four-chamber view shows a significantly dilated RA with sail-like anterior tricuspid leaflet (arrow). There is apical displacement of the septal tricuspid leaflet. LA: left atrium; LV: left ventricle; RA: right atrium; RV: right ventricle
CASE 3
CLINICAL PRESENTATION
A 14-year-old girl who was previously followed up at another centre for cardiac abnormality was referred for recurrent palpitations. Her blood pressure was 110/70 mmHg and heart rate was 80 bpm. Physical examination showed a well-thrived girl without evidence of cyanosis. There was a Grade 3 pan-systolic murmur that was loudest over the left lower sternal edge without any radiation. ECG was performed (
Fig. 5
ECG shows prominent delta waves consistent with Wolff-Parkinson-White syndrome.
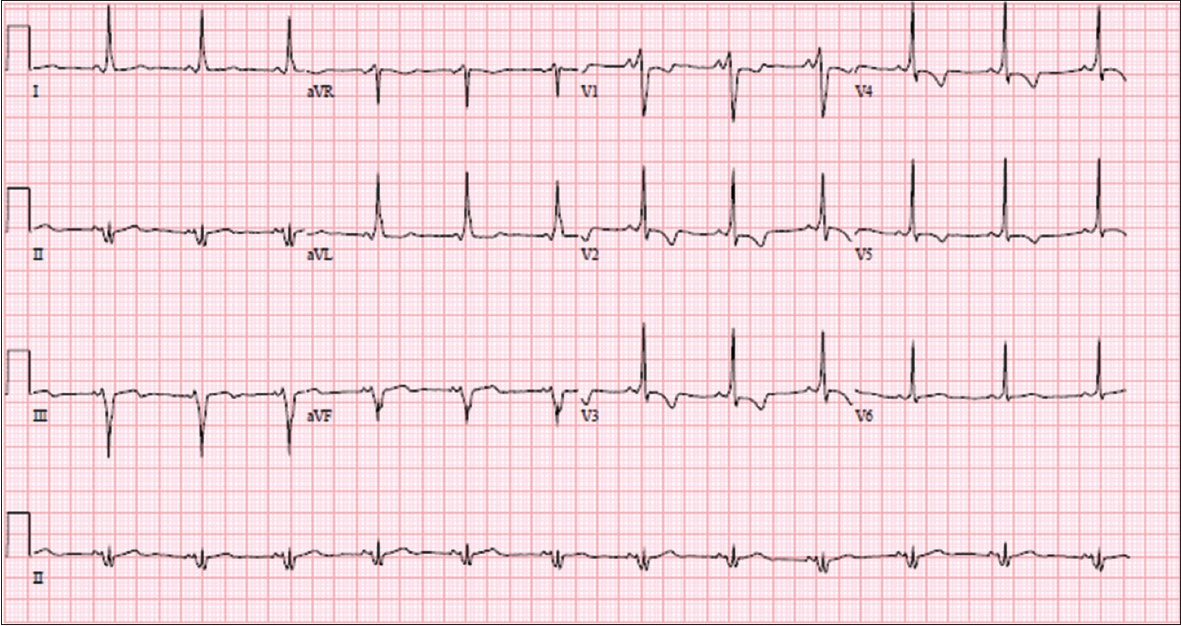
ECG INTERPRETATION
The ECG in
CLINICAL COURSE
Transthoracic echocardiography showed an Ebstsein’s anomaly malformation involving the tricuspid valve. There was a sail-like anterior leaflet and apical displacement of the septal leaflet by 31 mm/m2. There was non-coaptation of the tricuspid valve leaflets, resulting in severe TR. The RA and RV were dilated. Right and left ventricular ejection fraction was preserved. There was an associated secundum atrial septal defect.
The patient underwent an electrophysiology study that showed multiple right-sided accessory pathways. Successful radiofrequency ablation of multiple right-sided pathways was done. Subsequently, she successfully underwent a surgical cone repair of the tricuspid valve with bidirectional cavopulmonary connection.
DISCUSSION
Ebstein’s anomaly occurs in approximately one per 200,000 live births and accounts for < 1% of cases of congenital heart disease.(1,2) This anomaly was first described by Wilhelm Ebstein in 1866 in a report titled ‘Concerning a very rare case of insufficiency of the tricuspid valve caused by a congenital malformation’.(3) It is a congenital malformation characterised by malformed and displaced tricuspid valve leaflets that are partly attached to the tricuspid valve annulus and partly attached to the right ventricular endocardium. A wide range of tricuspid valve abnormalities are seen in Ebstein’s anomaly, ranging from minimal displacement of the septal and inferior leaflets to severe displacement with tethering. Management of Ebstein’s anomaly requires understanding of the anatomy and physiology along with clinical features. Although the diagnosis is largely based on echocardiographic features, ECG is an important tool.
Anatomy
The normal tricuspid valve has three leaflets: anterior, posterior and septal. In Ebstein’s anomaly, the septal and posterior leaflets are adherent to the underlying myocardium. There is apical displacement of the tricuspid valve leaflets, leading to dilatation of the atrialised portion of the RV. The anterior leaflet may have fenestrations and redundancy. There is marked dilatation of the anatomic tricuspid valve annulus. This results in a large chamber separating the true annulus from the functional RV, which is referred to as the ‘atrialised’ portion of the RV.(4,5)
The embryological basis is the failure of delamination of the septal, inferior and anterior leaflets of the tricuspid valve from the right ventricular myocardium.(3) Multiple cardiac defects are associated with Ebstein’s anomaly, including atrial or ventricular septal defects, patent foramen ovale, patent ductus arteriosus and pulmonary outflow tract obstruction.(3) Arrhythmias are common and include accessory pathways such as Wolff-Parkinson-White syndrome as well as atrial tachyarrhythmias.(6)
Pathophysiology
Reduced right heart contractility and presence of significant tricuspid regurgitation result in reduced forward flow of blood through the right heart. There is progressively severe dilatation of the RA and RV.
Clinical features
Clinical features are varied and depend largely on the anatomic severity. Most patients present in childhood, although a delayed presentation in adulthood can occur with less severe malformations. Neonates present with cyanosis and heart failure secondary to TR, while children and adults may present with features of cyanosis, heart failure, arrhythmias or paradoxical embolism.(7) On physical examination, patients may have a right ventricular heave and a pan-systolic murmur from TR. The first heart sound is widely split, and the tricuspid component is loud and delayed because of closure of the large anterior leaflet. The V wave of TR is seldom observed in the jugular pulse because of the damping effect of the large atrium, despite the presence of severe TR.(2)
Diagnostic evaluation
Electrocardiography
The ECG can be a useful tool in the diagnosis of Ebstein’s anomaly. Although ECG features may not be specific to the diagnosis, most patients with Ebstein’s anomaly have an abnormal ECG.(2) Characteristic ECG features in Ebstein’s anomaly include tall P waves (> 2.5 mm) in leads II and V1 due to right atrial enlargement.(7) In some cases, giant ‘Himalayan’ P waves have been described, defined as height > 5 mm.(8) Our patient in Case 1 had ECG features suggestive of right atrial enlargement.
PR interval prolongation with first degree AV block can be seen in 42% of patients.(2) This is attributed to prolonged conduction through an enlarged RA. Conversely, a short PR interval may be seen when associated with pre-excitation.(9) Our patient in Case 3 had a short PR interval associated with delta waves due to pre-excitation.
The QRS complex may be prolonged in Ebstein’s anomaly, due to prolonged activation of the RV. Incomplete or complete RBBB is seen in 44% of patients,(10) as seen in all three ECG cases. This happens due to a delay in conduction through the specialised conduction tissues.(11) Triphasic or tetraphasic QRS complexes can be observed. The QRS complex may be fragmented due to abnormal conduction through the atrialised RV, as was observed in all three cases. The finding of a giant P wave together with an atypical RBBB in V1 is suggestive of Ebstein’s anomaly.(10) The amplitude of the R and R’ in V1 is smaller than those in V5 and V6, contrary to a typical RBBB. Similarly, the presence of qR pattern in V1 also suggests an enlarged atrialised RV.(12)
Arrhythmias are another characteristic feature. Pre-excitation occurs more commonly in Ebstein’s anomaly than in any other congenital heart disease. Accessory pathways, when present, tend to be right-sided ones. Other arrhythmias in Ebstein’s anomaly include paroxysmal atrial flutter, atrial fibrillation and ventricular tachycardia.
Chest radiography
Chest radiographs may be normal in mild cases. In severe cases, a large globular heart is seen with a narrow waist. The right atrial silhouette may be enlarged. The term ‘box-shaped heart’ is used to describe some cases. Pulmonary vasculature may be normal or reduced.
Echocardiography
Transthoracic echocardiography is the diagnostic modality of choice for Ebstein’s anomaly. It is diagnosed based on apical displacement of the septal leaflet of the tricuspid valve by 8 mm/m2 or greater, combined with an elongated sail-like appearance of the anterior leaflet.(2) The atrium and atrialised portion of the RV are commonly markedly enlarged with significant TR. These features were found in all three cases described, some of which are shown in
Cardiac magnetic resonance imaging
Cardiac MR imaging offers further information into functional right ventricular volume and function, TR fraction and shunt quantification. It also allows for assessment of left ventricular size and function, left ventricular non-compaction, and regions of fibrosis on myocardial delayed enhancement imaging. The features of Ebstein’s anomaly on cardiac MR imaging can be seen in
Treatment
Medical management
Patients with Ebstein’s anomaly should be routinely followed up by a cardiologist with expertise in congenital heart disease. Management includes assessment for cyanosis and heart failure. Asymptomatic patients can be carefully monitored. Patients who develop heart failure and are not candidates for surgery can be treated with standard heart failure therapy, including diuretics. Patients with a history of paradoxical embolism or atrial fibrillation should be anticoagulated with warfarin. However, newer anticoagulants have not been specifically studied in the Ebstsein’s anomaly population. Options for management of arrhythmias include surgical or catheter ablation. Endocarditis prophylaxis before appropriate dental procedures should be considered in cyanotic Ebstein’s anomaly patients or those with a prosthetic cardiac valve.
Physical activity
Exercise recommendations are based on the American College of Cardiology’s Task Force I report on congenital heart disease. Patients with mild Ebstein’s anomaly with nearly normal heart size and no arrhythmias can participate in all sports. Those with severe Ebstein’s anomaly should not routinely participate in sports.(13)
Arrhythmias
Supraventricular arrhythmias are often seen in patients with Ebstein’s anomaly. Patients with symptomatic arrhythmias or those scheduled for surgical repair should undergo an electrophysiology study. The success rate for catheter ablation is lower in patients with Ebstein’s anomaly compared to those with structurally normal hearts.(14) Surgical interruption can be performed if catheter ablation is unsuccessful or inappropriate.
Surgery
Indications for surgery include symptoms such as decreasing exercise capacity, clinical features of cyanosis, paradoxical embolism, congestive cardiac failure and progressive RV dilation or reduction of RV systolic function.(15) Surgical techniques should be tailored to each patient, in discussion with a multidisciplinary team. Aims of surgery include improving the severity of TR, reducing the arrhythmia burden, closing any inter-atrial communications and pacemaker placement when necessary.(15) When possible, tricuspid valve repair is the preferred surgical method. Tricuspid valve replacement is reserved for valves not amenable to repair, or in patients with massive RV or tricuspid annular dilatation. Options for tricuspid valve replacement include mechanical or bioprosthetic valves. Operative management includes anti-arrhythmia procedures such as maze and ablation of accessory pathways.
Summary
Ebstein’s anomaly is a complex congenital condition with variable presentations. Recognition of clinical and ECG features can lead to improved diagnosis and management.
SMJ-60-565.pdf


