Abstract
Renal-related adverse effects of intravascular contrast media (CM) include contrast-induced nephropathy in computed tomography and angiography. While large retrospective studies have been published, the exact pathogenesis of this condition is still unknown. We review the main international guidelines, including the American College of Radiology white paper and the guidelines of European Society of Urogenital Radiology, Royal College of Radiologists and Canadian Association of Radiologists, as well as their references, regarding this subject. We present a simplified, concise approach to renal-related adverse effects of CM, taking into consideration the basis for each recommendation in these published guidelines. This will allow the reader to better understand the rationale behind appropriate patient preparation for cross-sectional imaging.
INTRODUCTION
In 1978, the first nonionic intravenous contrast medium (CM), i.e. metrimazide (Amipaque), was approved in the United States. Since then, the diagnostic capability of physicians, especially those in the fields of urology and cardiology, has been immensely revolutionised.(1) Following the increased use of intravenous iodinated CM, a higher number of CM-related adverse effects has been recognised, with contrast-induced nephropathy (CIN) constituting one of the most serious adverse effects.(2)
CIN is also known as contrast-induced acute kidney injury.(3) Nephrology literature on acute kidney injury have proposed several definitions and classifications for CIN.(4,5) However, the most widely quoted definition is from the Contrast Media Safety Committee of the European Society of Urogenital Radiology (CMSC ESUR), in which CIN is defined as a deterioration of renal function (defined as an increase in serum creatinine by more than 25% or 44 µmol/L) within three days of intravascular administration of CM in the absence of an alternative aetiology.(6)
CIN has been reported as the third most common cause of hospital-acquired renal failure, with an incidence rate of 11%.(7) A cohort study in a university hospital cardiac centre in Singapore showed a CIN incidence rate of up to 11.4% following intra-arterial administration under percutaneous coronary intervention (PCI).(8) CIN is also associated with an increased mortality rate of 9.7%.(9) Besides prolonging hospital stay,(10) CIN was shown to increase the incidence of a range of cardiovascular events, from coronary disease to stroke.(11)
With the rapidly ageing population and the increased prevalence of chronic kidney diseases (CKD), radiologists and referring physicians ought to be familiar with up-to-date and evidence-based practices of CIN. Each of the main international guidelines elaborates on different aspects of CIN management and at varying depths. Some guidelines adopt a slightly different approach from others, e.g. the management of metformin in patients with diabetes mellitus. In view of the complementary roles of international guidelines, this article aims to review and summarise the four main international guidelines: CMSC ESUR guidelines version 8.1; American College of Radiology (ACR) Manual on Contrast Media version 9 2013; Consensus Guidelines for the Prevention of Contrast Induced Nephropathy by Canadian Association of Radiologists (CAR) 2011; and Standards for Intravascular Contrast Agent Administration to Adult Patients by the Royal College of Radiologists (RCR) second edition 2010. The references within the aforementioned guidelines are also examined, with the related articles retrieved from the PubMed database.
PATHOPHYSIOLOGY OF CIN
All CM are rapidly distributed into intravascular and extracellular fluids following intravascular administration. They are solely eliminated by glomerular filtration.(7) Extrarenal excretion constitutes less than 1% in normal renal function.(12) It has been proven that approximately 100% of CM is excreted within the first 24 hours after administration in patients with normal renal function.(7) On the contrary, in patients with reduced renal function, the half-life of elimination can increase by up to 40 hours or more.(7)
Under physiological resting condition, 25% of the cardiac output is directed to the kidneys.(7) The majority of vascular flow is channelled toward the cortex to optimise glomerular filtration and reabsorption of water and salts.(7) Renal medullary blood flow is, on the contrary, low.(7,13) This makes the renal medulla prone to ischaemic injury, cellular damage and consequently, CIN.(14) The exact pathophysiology of CIN is not well understood. The accepted aetiologic factors comprise three different but potentially interacting pathways: the haemodynamic effects of CM; the effects of reactive oxygen species (ROS); and direct tubular cellular toxicity by CM molecules.(7,15)
Haemodynamic effects
The deeper portion of the outer medulla of the kidney is metabolically active; hence, it is predisposed to hypoxic injury.(7) Following administration of CM, a biphasic haemodynamic response occurs – a brief initial phase of increased renal blood flow, followed by prolonged flow reduction by 10%–25% below the baseline. This decreases the partial pressure of oxygen (PO2) of the outer medulla by 50%–67% to the level of 9–15 mmHg (compared to 20 mmHg under physiological condition). Furthermore, the higher the osmolality of the CM, the higher the oxygen requirement of the tubular cells, making them even more vulnerable to hypoxic injury and CIN.(14)
Effects of ROS
Medullary hypoxia following CM administration also leads to an increased formation of ROS.(7) These ROS include superoxide (O2−), hydroxyl radicals (OH−) and less aggressive reacting molecules such as hydrogen peroxide (H2O2).(15,16) Once exceeding the scavenging capabilities of antioxidants, these ROS cause oxidative stress and lead to ischaemia reperfusion injury at the cellular level.(7) ROS also triggers and increases angiotensin II- and endothelin I-induced vasoconstriction, and decreases the bioavailabilty of vasodilative nitric oxide (NO), thus compromising the ischaemic state of the outer medulla. This whole process forms a vicious cycle, leading to CIN.(17)
Effects of tubular cell toxicity
Other effects of CM on tubular cells include intercellular junction disruption, membrane protein redistribution, DNA fragmentation, reduction of extracellular Ca2+, and even altered mitochondrial function.(18) High osmolality CM (HOCM) has been shown to produce a more pronounced toxic effect than low- or iso-osmolar CM,(7) but HOCM is fortunately not used in current practice. Historically, sodium acetrizoate (Urokon®) was the first ionic HOCM that was synthesised in 1953, followed by sodium diatrizoate (Urografin®) and sodium iothalamate (Conray®). Due to the serious adverse effects from the high osmolality and ionicity, HOCM have been replaced by low osmolality CM (LOCM).
In summary, CIN occurs as a result of medullary ischaemia following both increased oxygen consumption by tubular cells and decreased perfusion in the renal outer medulla. These involve interacting processes of ROS formation, imbalance between vasoconstrictive and vasodilatative mediators, and direct toxicity on the tubular cells.(7)
MAIN PREDICTOR OF CIN – RENAL IMPAIRMENT
The most important predictor of CIN is renal impairment.(3) Renal impairment increases the risk of CIN by more than 20 times.(19,20) In patients with normal renal function, the development of CIN after intravascular CM is extraordinarily rare, or does not occur at all.(3,19) With declining renal function, there is a corresponding increased risk of CIN.(6) Numerous studies have shown that the incidence of CIN varies from less than 2% in the general population to up to 50% in patients with advanced renal diseases.(7)
Conventionally, in patients receiving intra-arterial injection, a cautious threshold of estimated glomerular filtration rate (eGFR) < 60 mL/min is practised.(6) This route of CM administration is more commonly seen in cardiovascular angiography and vascular interventional radiology. Consensus guidelines from CAR classify this category of patients as moderate to high risk. This is because intra-arterial injection is associated with at least twice the risk of CIN and poorer outcomes compared to the intravenous route of administration.(3) This practice is supported by numerous radiology literature; for instance, Moore et al’s study involves a randomised double-blinded clinical trial comparing between groups of patients who underwent angiocardiography and computed tomography (CT), which showed a relative odds of 3.44.(21)
One recent major change to eGFR cutoffs is outlined in the updated guidelines from CMSC ESUR.(6) For patients receiving intravenous CM, the precautionary cutoff level has been lowered from eGFR < 60 mL/min to eGFR < 45 mL/min. This is because data and review of intravenous CM administration studies have shown that the risk of CIN increases only when eGFR is < 45 mL/min. This is further supported by a study by Katzberg and Barrett in 2007, which showed that the incidence of CIN in patients with eGFR < 45 mL/min varied between 5% and 20% as compared to less than 1% in those with eGFR > 45 mL/min.(22)
Manuals from ACR and guidelines from RCR have yet to discriminate between intra-arterial and intravenous routes of administration for CIN risk assessment.(19,23) However, ACR manuals have mentioned that the higher overall incidence of CIN reported in several publications(24) is likely an overestimation among patients undergoing intravenous contrast-enhanced studies.(19) RCR guidelines state that the eGFR level chosen to trigger special precautions may be set locally after discussion between local radiologists and nephrologists.(23)
OTHER RISK FACTORS FOR CIN
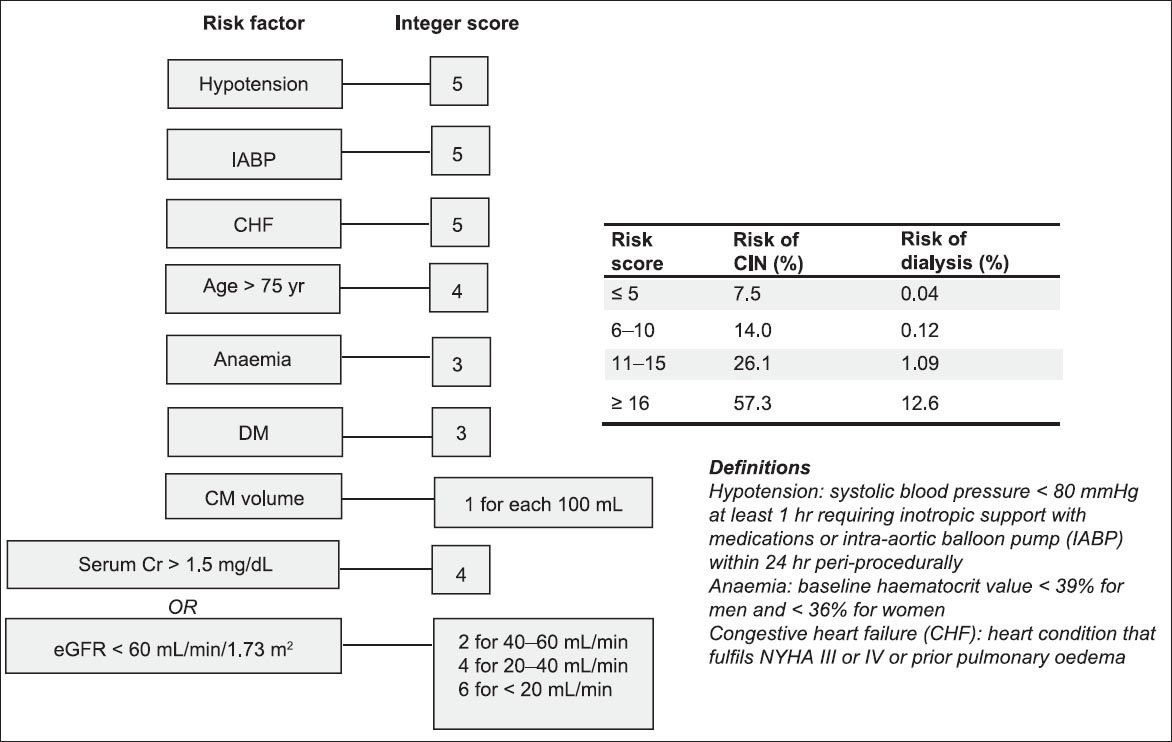
Apart from renal impairment, comorbidities are equally important risk factors for CIN, as the risk and severity of CIN increases proportionally with the number and severity of risk factors.(6) In 2004, Mehran et al(25) introduced a simple scoring system for the prediction of CIN risk after PCI, which involves eight risk factors, as shown in
Fig. 1
Mehran’s scoring system.(25) CIN: contrast-induced nephropathy; CM: contrast media; Cr: creatinine; DM: diabetes mellitus; eGFR: estimated glomerular filtration rate; NYHA: New York Heart Association
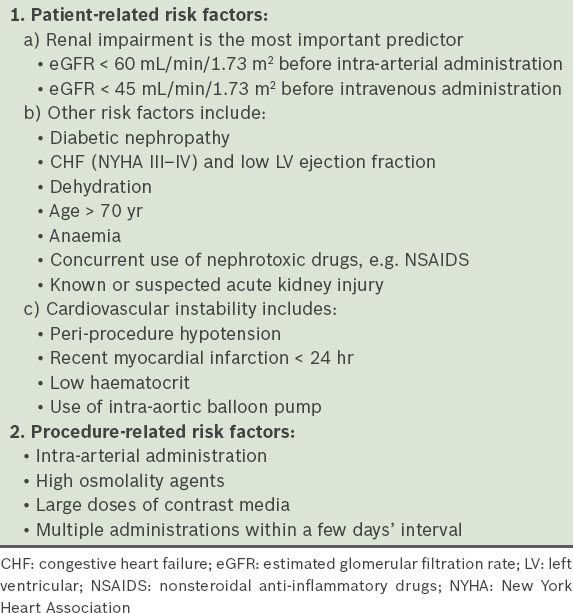
ESUR guidelines categorise risk factors for CIN as patient- or procedure-related.(6) Patient-related risks encompass diabetic nephropathy, dehydration, congestive cardiac failure (New York Heart Association [NYHA] II–IV), low left ventricular ejection fraction, gout, age > 70 years, concurrent administration of nephrotoxic drugs, e.g. non-steroidal anti-inflammatory drugs (NSAIDs), and known or suspected acute renal failure. The list of patient-related risk factors has been expanded to include factors related to cardiovascular instability, such as periprocedural hypotension, recent myocardial infarction (< 24 hours), low haematocrit level and use of an intra-aortic balloon pump. Procedure-related risks are related to intra-arterial route of administration, high osmolality agents, large dosage and multiple administrations within an interval of a few days.(6) These risk factors are summarised in
Table I
Risk factors for contrast-induced nephropathy in intravenous iodinated contrast agents based on the European Society of Urogenital Radiology guidelines.(6)
Consensus from CAR includes the following as part of the contributing factors of CIN: sepsis; previous chemotherapy; organ transplants; human immunodeficiency virus; and collagen vascular diseases.(3) In addition, ACR manuals incorporate hyperuricaemia as one of the risk factors for CIN.(19) Studies by Okino et al(26) in 2010 and Saritemur et al(27) in 2013 demonstrated hyperuricaemia as an early predictor of slow and mild development of renal insufficiency after PCI.
SCREENING OF SERUM CREATININE BEFORE INTRAVASCULAR CM ADMINISTRATION: IS IT NECESSARY?
Routine measurement of serum creatinine and eGFR is not practical, and may delay scheduled investigations, disrupt bookings and incur extra costs.(28,29) The validity of an available serum creatinine level for CIN risk assessment can range from one week to six months, depending on the pre-existing guidelines or manuals.(3,6,19,23)
CAR consensus suggests the measurement of serum creatinine and eGFR within six months in stable outpatients with one or more risk factors but without significant renal impairment.(3) More recent serum creatinine measurement (within an interval of one week) is needed for inpatients and patients with unstable or acute renal disease.(3) RCR guidelines corroborate with the CAR consensus, but recommends a shorter duration of three months for patients with stable clinical condition. A ‘recent’ measurement is recommended for diabetic patients or patients requiring intra-arterial injection, although no specific interval was outlined. A risk-versus-benefit approach has to be applied in acutely or severely unwell patients, such as those with hypotension or hypovolaemia.(23)
According to the ESUR guidelines, serum creatinine (and eGFR) measurement within one week’s interval is required for patients with known eGFR < 60 mL/min/1.73 m2, patients who require intra-arterial CM, patients aged > 70 years, patients with a history of diabetes mellitus, hypertension (not necessarily requiring medical therapy as opposed to ACR guidelines), gout, renal disease, renal surgery, proteinuria, or who were on recent nephrotoxic drugs.(6) The ACR manuals state that there is no universally accepted interval between the baseline serum creatinine measurement and CM administration. Some have accepted a 30-day interval as adequate, with a shorter interval for inpatients and those with new risk factors for renal dysfunction.(19)
In routine clinical practice, we frequently encounter outpatients who present for contrast-enhanced studies without a baseline serum creatinine level. CIN risk assessment is therefore limited, and options include an alternative noncontrast study or on-the-spot serum creatinine measurement. This has resulted in delayed clinic appointment, patient dissatisfaction, extra administrative work, greater time consumption, and even compromised radiological analysis if plain cross-sectional imaging is opted.(30) Choyke et al(30) supplemented this gap in their study, which found that patients who had abnormal serum creatinine level with high specificity could be excluded from serum testing prior to contrast injection for imaging studies. Patients in the study completed a questionnaire, which included questions on the presence of the following risk factors: pre-existing renal dysfunction; proteinuria; prior renal surgery; hypertension; diabetes mellitus; and gout. The study suggested that if all six of these survey questions were answered in the negative, 94% of patients would have a normal serum creatinine level and 99% would have a serum creatinine level under 1.7 mg/dL (150.3 µmol/L). Thus, the authors concluded that patients without the aforementioned risk factors could reasonably be excluded from serum creatinine screening prior to contrast injection.(30)
It is worth emphasising that serum creatinine is not a reliable indicator of renal function, as the normal serum creatinine level is usually maintained until the GFR is reduced by nearly 50%.(19) According to the Modification of Diet in Renal Disease (MDRD) or Cockcroft-Gault formulae, eGFR in adults is widely accepted as an index of renal function.(19) However, one should bear in mind that the MDRD formula is known to underestimate eGFR in patients with normal or near-normal renal function.(31) The formula is intended for use in patients with CKD. It is not designed to monitor acute changes in renal function, and therefore, does not perform well in ill, hospitalised patients, who make up the majority that require radiological imaging.(32)
METFORMIN AND INTRAVASCULAR CM USE
With a rising trend of metformin use among patients with diabetes mellitus, radiologists and clinicians should be well versed with metformin management during the period of CM administration. Approximately 90% of the administered metformin is eliminated via the kidneys in 24 hours.(12) Metformin itself does not confer an increased risk of CIN.(19) Instead, it carries a very rare risk of lactic acidosis in patients with renal failure.(19)
PREVENTIVE MEASURES OF CIN
CIN usually manifests as transient asymptomatic elevation of serum creatinine, which begins within 24 hours of contrast injection, peaks within 4 days, and returns to baseline within 7–10 days.(33) It is not commonly associated with permanent renal dysfunction.(19) Despite a self-limiting clinical course in most cases,(6) prevention is crucial to avoid increased morbidity and mortality.(7) The majority of the international guidelines practise the same preventive measures in reducing the risk of CIN. These will be discussed in-depth in the relevant sections and systematically outlined as ‘before’, ‘during’ and ‘after’ the procedure.
Before the procedure
The first step to preventing CIN is to identify at-risk patients. These patients should be considered for alternative imaging modalities that do not require CM injection, such as ultrasonography or magnetic resonance (MR) imaging. There is no universally agreed upon threshold of serum creatinine elevation that contraindicates the administration of CM. ACR manuals state that there is insufficient data at this time to prescribe a specific recommended threshold.(19) However, it is believed that the risk of CIN is sufficiently low for eGFR > 45mL/min and is likely to be safe for most patients.(22) The bottomline practice lies in weighing the risks and benefits.(23) There may also be a need to discuss with the referring physician to stop any nephrotoxic drugs, e.g. stop NSAIDs for at least 24–48 hours.(6)
Fluid volume expansion and avoidance of dehydration are the mainstays of CIN prevention. The principle of volume expansion is to increase intravascular volume, renal blood flow and diuresis, reduce the contact time of CM with renal tubular cells, and suppress the renin-aldosterone system.(7) Controversy exists in the types and routes of hydration, as well as the optimal duration required. A simplified approach to CIN prevention is outlined in
Table II
Simplified preventive measures to contrast-induced nephrology in computed tomography.

Type of fluid regime: 0.9% vs. 0.45% of normal saline
Both the ESUR and ACR guidelines unanimously agree that 0.9% saline is superior to 0.45% saline in the reported risk of CIN reduction.(6,19) This is supported by studies conducted by Weishord et al and Mueller et al in the year 2008 and 2002, respectively.(34,35) However, the infusion rate varies according to different guidelines. ESUR guidelines suggest an infusion rate of 1.0–1.5 mL/kg/hr for at least 6 hours before and 6 hours after CM injection.(6) CAR consensus recommends a similar rate of 1.0 mL/kg/hr but for a longer duration of 12 hours pre- and post-procedure.(3) ACR manuals, on the other hand, recommend a higher infusion rate of 100 mL/hr for 6–12 hours before and 4–12 hours after CM injection,(19) while RCR guidelines do not commit on the infusion rate and interval of hydration.(23)
Type of fluid regime: 1.4% of sodium bicarbonate vs. 0.9% of normal saline
There is ongoing debate on the use of 1.4% of sodium bicarbonate in CIN risk reduction. 154 mEq/L of sodium bicarbonate diluted in dextrose 5% water is an alternative fluid protocol. ESUR guidelines suggest 3 mL/kg/hr of 1.4% sodium bicarbonate for 1 hour before and 1 mL/kg/hr for 6 hours after CM injection. This aims to enhance the alkalisation of renal tubular fluid and suppress production of ROS.(36) Merten et al,(37) who published the first randomised controlled trial (RCT) on this subject, described 119 patients who were randomly assigned to receive either NaCl 154 mEq/L in 5% dextrose/H2O or NaHCO3 154 mEq/L in 5% dextrose/H2O. The study showed convincing evidence in favour of NaHCO3 hydration, with 1.7% of CIN incidence in the NaHCO3 group compared to 13.6% in the NaCl group.(37)
Two subsequent RCTs by Recio-Mayoral et al in 2007 and Pakfetrat et al in 2009 compared the effect of a single bolus of NaHCO3 before coronary angiography or PCI; both studies showed a significant reduction of CIN in the group treated with the NaHCO3 bolus.(38,39) One meta-analysis involving a total of 1,734 patients showed that NaHCO3 is superior to NaCl alone in the prevention of CIN in patients with moderate to severe CKD.(40) However, a study of 353 patients undergoing coronary angiography (MEENA trial) showed no benefit of sodium bicarbonate over normal saline in preventing CIN.(41) ACR guidelines also state that sodium bicarbonate cannot be considered definitive at this period of time, as it is challenged by a meta-analysis conducted by Zoungas et al in 2009.(42)
Route of hydration: oral vs. intravenous
Intravenous fluid is the preferred route of hydration due to better control of volume expansion.(7) According to ESUR, oral fluid of 1,000 mL over 6–8 hours before and after contrast exposure could be sufficient for patients with eGFR of 30–45 mL/min and receiving intravenous CM of ≤ 100 mL.(43) CAR consensus states that oral hydration is not an evidence-based substitute for intravenous hydration, although some institutions might recommend it in certain outpatients due to the impracticality of intravenous hydration.(3) It was mentioned in the ACR manuals that oral hydration has been utilised but with less demonstrated effectiveness.(19)
N-acetylcysteine: is it useful?
N-acetylcysteine (NAC) was once widely advocated in patients at risk of CIN following an initial publication by Tepel et al in the year 2000.(44) The typical regime consists of 600 mg of NAC administered orally for two days prior to the procedure.(44) Increasing evidence, however, suggests that NAC is not efficacious in CIN prevention.(45,46) The most meticulous meta-analysis, conducted by Gonzales et al in 2007, also found no beneficial role of NAC in CIN risk reduction.(47) However, based on its ease of use and lack of side effects, many institutions may still opt to add it to a renal protection protocol.(3,43) However, this should not be considered a substitute for hydration.(3,43)
Other drugs
Many other drugs have also been postulated to play a role in CIN prophylaxis. These include furosemide, mannitol, fenoldopam, dopamine, atrial natriuretic peptide, calcium channel blocker, L-arginine, endothelin receptor blocker and prostaglandin E1.(48-51) However, the available evidence for the use of these drugs is not convincing,(49) and some have even resulted in harmful side effects to the patients.(49) Potential drugs like theophylline or aminophylline, statins, ascorbic acid, iloprost and nitrates may still require further evaluation.(52-54) Both the ESUR and ACR guidelines opine that renal vasodilators, receptor antagonists of endogenous vasoactive mediators and cytoprotective agents have no consistent protective role against CIN; hence, these agents are not recommended.(6,19)
During the procedure
To reduce the risk of CIN, the majority of the guidelines unanimously recommend the use of low-osmolar CM (e.g. iohexol; Omnipaque®) or iso-osmolar nonionic CM (e.g. iodixanol; Visipaque®), with the lowest dose consistent with a diagnostic result.(3,6,19,23) Nevertheless, the available evidence fails to establish a clear advantage of intravenous iso-osmolar iodixanol over intravenous low-osmolar CM with regard to CIN.(55)
In general, patients with eGFR < 60mL/min should preferably receive an amount of CM of not more than 100 mL in volume.(56) A volume limit of 5 mL/kg of body weight normalised to the concentration of serum creatinine has been proposed as the threshold of CIN in CKD patients.(57) A study by Laskey et al suggests that a CM volume to creatinine clearance (CrCl) ratio of 3.7 can prospectively determine the maximum volume of CM without substantially increasing the risk of CIN; a higher risk is seen in patients receiving CM greater than the ratio of 3.7.(58) A most recent meta-analysis has also proven that reduction of CM volume with the use of automated contrast injector significantly reduces the incidence of CIN in patients undergoing coronary angiography.(59) However, according to ACR manuals, there is a lack of robust data to support a dose-toxicity relationship for intravenous iodinated CM, as only intracardiac iodinated CM shows a directly proportional relationship.(19)
MR imaging is an alternative imaging modality when CT cannot be performed. However, when intravascular CM needs to be administered, none of the international guidelines suggested replacing CT with MR imaging, since gadolinium is also contraindicated in renal-impaired patients.(60) In patients with eGFR < 30 mL/min, gadolinium carries the risk of a rare and potentially fatal disease known as nephrogenic systemic fibrosis (NSF).(61) According to experimental studies, this disease is due to an activation of circulating fibroblasts following cytokines released by skin macrophages and peripheral blood monocytes.(62) The exact pathogenesis of NSF is unknown. However, it has been postulated that NSF is possibly a result of gadolinium released from its chelates, which subsequently binds to phosphate, causing deposition in the skin and subcutaneous tissues.(62)
In general, those with eGFR < 30 mL/min are advised to avoid both iodinated intravenous CM and intravenous gadolinium to conserve the residual renal function and reduce the risk of NSF, respectively.(6,19) Recommendations include alternative imaging procedures or imaging without the use of intravenous CM. Fortunately, with the introduction of macrocyclic gadolinium chelate agents, the risk of NSF has now been largely reduced. This is attributed to the very high complex stability of macrocyclic agents, which decreases the risk of gadolinium ions released in vivo.(63) Some examples of macrocylic agents include gadoterate meglumine (Dotarem), gadobutrol (Gadovist) and gadoteridol (Prohance).(19) After weighing the risks and benefits, if intravenous gadolinium is still deemed absolutely necessary, avoidance of category one gadolinium (e.g. gadodiamide [Omniscan], gadopentetate dimeglumine [Magnevist], gadoversetamide [Optimark]) is advised.(19)
After the procedure
Management of CIN risk does not stop at the completion of contrast-enhanced studies. Volume expansion therapy should continue and eGFR values at 48–72 hours after the procedure should be obtained. These are clearly outlined in the ESUR guidelines.(6) According to the CAR guidelines, repeated use of CM should be avoided within 48–72 hours after the first administration, as a significantly increased risk of CIN has been demonstrated among patients who received a second dose of CM within 48 hours.(3,64) ACR manuals do not object to the practice of avoiding repeated CM injection within 24 hours.(19)
ROLE OF PROPHYLACTIC DIALYSIS OR HAEMOFILTRATION IN CIN
According to the CAR consensus, dialysis does not play a prophylactic role in reducing the risk of CIN.(3) Administered CM reaches the kidneys within 1–2 cardiac cycles, making it biologically implausible for removal by dialysis.(3) It is also unlikely to halt the initiated cascade of renal injury when CM has reached the kidneys.(3) The ESUR guidelines do not discuss the prophylactic role of dialysis in patients receiving intravascular CM, likely due to the abovementioned reason.(6) However, it clearly points out that there is no evidence to support the protective role of haemodialysis in CIN.(6) A meta-analysis by Cruz et al in 2006, which included six RCTs and two non-RCTs, demonstrated no additional benefit of peri-procedural extracorporeal blood purification compared to the standard medical therapy.(64)
CIN IN PATIENTS ALREADY ON DIALYSIS
It has been well established that dialysis patients are not contraindicated to intravenous iodinated CM administration.(6,19) ACR guidelines state that intravenous CM administration has a theoretical risk of causing a dialysis patient to change from an oliguric state to an anuric state, but this remains speculative, with no conclusive data seen.(19) Dialysis patients are also at a theoretical risk of osmotic load from intravenous iodinated CM, as they are unable to remove the imposed excessive intravascular volume.(19) The CM dosing should therefore be as low as necessary.(19) There is no need for an urgent dialysis unless an unusually large volume of CM is used, or there is a substantial underlying cardiac dysfunction.(65) ESUR guidelines also state that it is not necessary to correlate the time of CM injection with haemodialysis sessions, or to have extra haemodialysis sessions.(6)
CONCLUSION
Despite the numerous retrospective studies published, the exact pathogenesis of renal-related adverse effects of intravascular CM has yet to be completely understood. Nevertheless, every healthcare personnel should be aware of CIN, as at-risk patients have become increasingly more common. While the exact pathogenesis of CIN is unknown, interventions must be put in place. In patients receiving intravenous iodinated CM, the previously accepted threshold of eGFR < 60 mL/min has been lowered to eGFR < 45 mL/min, while a threshold of eGFR < 60 mL/min remains for CM delivered via the intra-arterial route.
To reduce the risk of CIN, hydration remains the mainstay of CIN prophylaxis in at-risk groups, and should continue into the post-procedural period. The benefits of sodium bicarbonate and NAC have been put to question in recent meta-analyses. Overall, the use of intravascular CM should be clinically justified and balanced between the risks and benefits of use, with consideration of alternative imaging modalities.


