Abstract
We report a case of a 61-year-old woman with a large atrial septal defect (ASD) that was detected incidentally on chest radiography and computed tomography when she presented with sepsis. Echocardiography confirmed a large secundum ASD with left-to-right shunt flow, right heart dilatation and severe pulmonary hypertension. The patient had a poor clinical outcome despite intensive care and eventually passed away. Haemodynamically significant ASDs have a known association with increased morbidity and mortality, and their early detection and closure cannot be understated. This article aimed to highlight the imaging features of ASD, with special emphasis on the routine chest radiograph. The pathophysiology and clinical manifestations of ASD are also briefly discussed.
CASE PRESENTATION
A 61-year-old Chinese woman presented to the emergency department with fever, vomiting and anorexia of three days’ duration. She had a past medical history of hypertension, hyperlipidaemia and a previous cerebrovascular accident.
On examination, the patient was toxic looking, confused and jaundiced. Her vital signs were as follows: temperature 40.5°C, blood pressure 134/67 mmHg, respiratory rate 20 breaths per minute, pulse rate 149 beats per minute and oxygen saturation 98% on room air. Physical examination revealed soft S1 and loud S2 heart sounds, with no cardiac murmur. There were crepitations in the lung bases and tenderness over the right hypochondrium. Initial blood investigations showed leucocytosis, conjugated hyperbilirubinaemia, and elevated alkaline phosphatase and transaminase levels. Electrocardiography showed right bundle branch block with right axis deviation.
Chest radiography (
Fig. 1
Frontal chest radiograph.
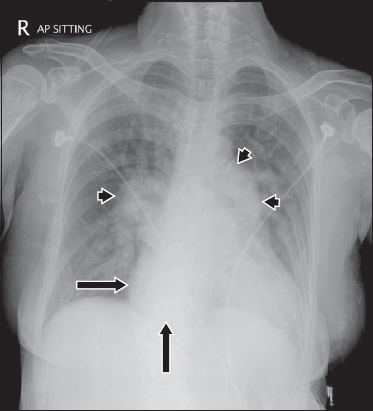
Fig. 2
Coronal contrast-enhanced CT image of the abdomen.
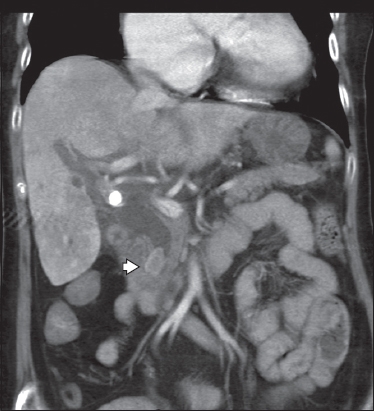
IMAGE INTERPRETATION
Anterior-posterior chest radiograph (
The CT images of the abdomen and pelvis show biliary obstruction secondary to a calculus in the distal common bile duct (arrowhead,
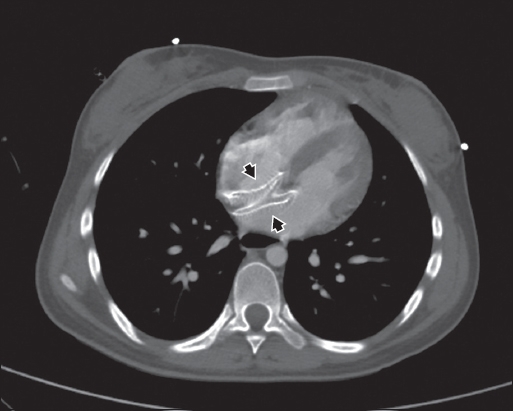
Fig. 3
Axial contrast-enhanced CT image of the appended lower chest.
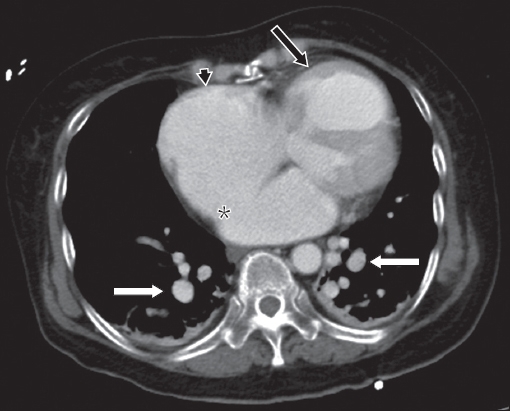
DIAGNOSIS
Atrial septal defect with pulmonary arterial hypertension.
CLINICAL COURSE
The patient required intubation and intensive care on admission. She also developed complications of Gram-negative septicaemic shock, pneumonia and acute kidney injury. A cardiologist reviewed her in the intensive care unit. Echocardiography that was performed confirmed a large secundum ASD with left-to-right shunt flow, severe right ventricular systolic dysfunction, severe tricuspid regurgitation and severe pulmonary hypertension. Pulmonary artery systolic pressure (PASP) was 70 mmHg and left ventricle ejection fraction was 55%. She was unlikely to have Eisenmenger’s syndrome, as her oxygen saturation level was normal on room air.
The patient initially responded to the treatment of hydration, intravenous antibiotics and biliary stenting. However, her condition subsequently deteriorated when she developed critical illness neuropathy and recurrent desaturations. She eventually passed away.
DISCUSSION
Our patient was incidentally diagnosed with ASD in late adulthood when she presented with hepatobiliary sepsis. The presence of a large ASD with severe pulmonary hypertension, in this case, was deemed a significant contributor to her poor clinical outcome. ASD can present at any age and is also the most common congenital heart disease presenting in adulthood.(1,2) This article aimed to highlight the imaging features of ASD, with special emphasis on the routine chest radiograph. The pathophysiology and clinical manifestations of ASD were also briefly discussed.
An ASD is an abnormal communication between the left and right atria of the heart. It produces a constant left-to-right interatrial shunt flow and also intermittent right-to-left shunt flow from transient increases in right atrial pressure during a Valsalva-like activity such as forceful coughing. This right-to-left shunt flow can result in paradoxical embolisation. A patent foramen ovale, on the other hand, produces only intermittent right-to-left interatrial shunt flow.(3) Constant left-to-right shunting due to the ASD may result in right heart dilatation and pulmonary arterial hypertension over time. The magnitude and direction of shunting are related to the defect size and relative diastolic filling properties of the left and right ventricles.(2) For example, a left-to-right shunt is increased with left ventricular hypertrophy or mitral stenosis.(4) The majority of adults with ASDs remain asymptomatic. Early signs may be picked up on health screening examinations, electrocardiography or chest radiography. Auscultation may reveal a faint to moderately loud systolic ejection murmur best heard at the upper left sternal border; wide split fixed S2; absent thrill; and a barely audible to faint diastolic flow rumble at the lower left sternal border.(4) When patients with ASDs eventually become symptomatic, they may commonly present with symptoms such as exertional dyspnoea, decreased effort tolerance and palpitations from arrhythmias.(2)
Radiographic evidence of ASD is often subtle. The normal cardiomediastinal outline is reviewed in
Fig. 4
Normal frontal chest radiograph shows the right and left mediastinal outlines. The right mediastinal outline is formed by the superior vena cava (cyan dashed line), right pulmonary artery (black solid line), right atrium (green dashed line) and inferior vena cava (blue dashed line). The left mediastinal outline is formed by the aortic knuckle (red dashed line), main pulmonary artery (white dashed line), left pulmonary artery (white solid line), left atrial appendage (yellow dashed line) and left ventricle (magenta dashed line). The right atrium should only show mild convexity. The normal size of the pulmonary arteries has been likened to the little fingers of adults.
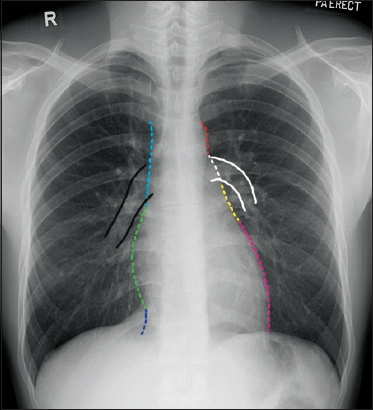
On the chest radiograph, the first sign of underlying ASD is right heart dilatation. Increased convexity and enlargement of the right heart border suggest right atrial dilatation. When the right ventricle dilates, the left ventricle is displaced superolaterally, which results in increased convexity of the left heart border and cardiac apex.(6) Long-standing ASD results in pulmonary hypertension (PH), which is an abnormal elevation of pressure in the pulmonary circulation (mean pulmonary arterial pressure > 25 mmHg). Features of PH on chest radiographs include central pulmonary artery dilatation (due to increased pulmonary flow), pruning of the peripheral arteries and increased diameter of the right interlobar artery (15 mm in women and 16 mm in men), as measured from its lateral aspect to the interlobar bronchus. In addition, a small aortic knuckle can be seen in patients with ASD, a manifestation of decreased systemic flow.
There are many causes of PH and it is important to look for clues to the underlying causes on chest radiographs, such as interstitial lung disease, emphysema and chest wall deformities. The Fifth World Symposium on Pulmonary Hypertension held in Nice, France, in 2013 updated the clinical classification of PH in the following groups: disorders that cause pulmonary arterial hypertension (Group 1); PH due to left heart disease (Group 2), PH due to chronic lung disease or hypoxia (Group 3); chronic thromboembolic pulmonary hypertension (Group 4); and PH due to unclear multifactorial mechanisms (Group 5).(7) The classification of PH is listed in
Table I
Classification of pulmonary hypertension, adapted from the Dana Point classification system.(7)
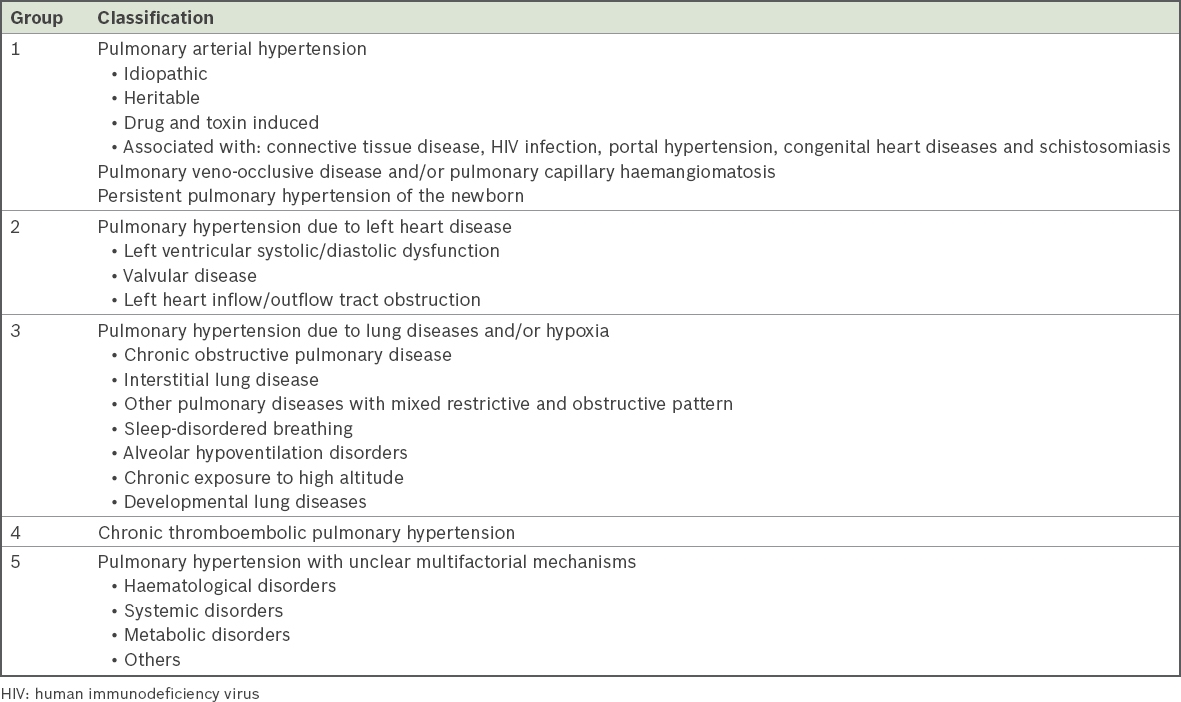
Fig. 5
A 19-year-old woman presented with shortness of breath on exertion and palpitations. Frontal chest radiograph shows dilated central pulmonary arteries (white arrows) with a small aortic knuckle (white arrowhead), which suggests a left-to-right shunt. There is also right atrial dilatation (black arrowhead) and elevation of the cardiac apex (black arrow) due to right ventricular dilatation. A large secundum atrial septal defect (ASD; 29 mm × 35 mm) and elevated pulmonary artery systolic pressure (PASP) of 44 mmHg were seen on echocardiography (not shown).
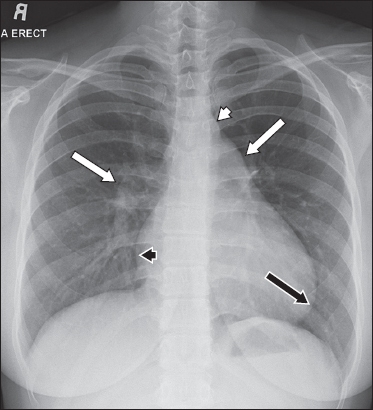
Fig. 6
A 46-year-old lady presented with palpitations. Frontal chest radiograph shows dilatation of the right atrium (black arrowhead) and central pulmonary arteries (white arrowheads). A large secundum ASD (25 mm × 30 mm) and elevated PASP of 52 mmHg were seen on echocardiography (not shown).
The diagnostic modality of choice for ASD is echocardiography, as it provides both structural and functional information.(8) Transthoracic echocardiography may be limited in assessing the right ventricle due to poor acoustic windows in some patients. Transoesophageal echocardiography may be performed to obtain a better acoustic window, but it is invasive in nature. Cardiovascular magnetic resonance (CMR) imaging, CT and diagnostic cardiac catheterisation are alternative modes of imaging. CMR imaging is the gold standard for the assessment of right ventricular volumes and function. It also allows for shunt quantification (Qp/Qs ratio; ratio of total pulmonary blood flow to total systemic blood flow) by using velocity flow mapping. A Qp/Qs ratio of 1:1 is normal and usually indicates that there is no shunting. CT and CMR imaging both have the advantage of allowing the visualisation of pulmonary venous return, which is especially important in excluding a concomitant partial anomalous pulmonary venous drainage.
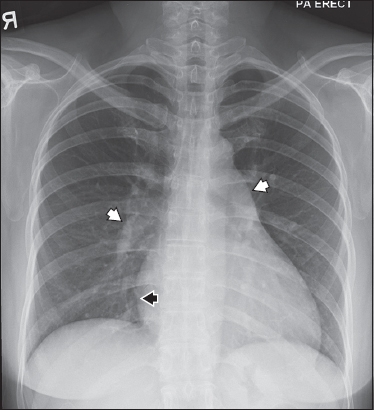
Haemodynamically significant ASDs are associated with increased morbidity and mortality.(2) Early detection and closure of significant ASDs can help reduce morbidity by preventing some of the associated complications. These complications include arrhythmias, paradoxical embolism, stroke, brain abscess, pulmonary hypertension, pulmonary artery aneurysm, right ventricle failure and Eisenmenger’s syndrome.(8,9) Transcatheter device closure of suitable secundum ASDs (
Fig. 7
A 20-year-old woman was asymptomatic with an incidental finding of a heart murmur. Echocardiography (not shown) revealed a large secundum ASD (20 mm × 20 mm), elevated PASP of 41 mmHg, and mitral valve prolapse, which was the cause of the murmur. She underwent ASD closure with an Amplatzer septal occluder (area between black arrowheads). Frontal chest radiograph shows the typical double-disc configuration of the Amplatzer septal occluder device. There are also features of dilatation of the right heart and pulmonary arteries.
Fig. 8
Axial contrast-enhanced CT image of the same patient from
SMJ-59-283.pdf
ACKNOWLEDGEMENTS
The authors would like to acknowledge Dr Lynette Teo, Dr Lee Chin Hwee and all colleagues at National University Hospital, Singapore, who were involved in the care of our patient.


