Abstract
INTRODUCTION
Vertical transmission of the hepatitis B virus (HBV) is higher in infants born to pregnant women with a higher HBV DNA viral load even if the infants complete both active and passive vaccination. Although antiviral treatment is recommended for pregnant women during the antenatal period to reduce the rate of vertical transmission, most of them decline treatment.
METHODS
A decision tree was made to evaluate the costs and benefits involved when pregnant women either agreed or declined to take antiviral treatment during the antenatal period. The cost-effectiveness price was arrived at by multiplying the reduced vertical transmission rate with expenses of future medical care associated with vertical transmission.
RESULTS
From an individual mother’s perspective, it was not cost-effective to receive antenatal antiviral treatment given the observed medication price and transmission rate in Singapore. However, the health system asserts that the current price of antiviral treatment is already far below the cost-effectiveness level, even without the Ministry of Health subsidies. Additionally, the awareness and perception of pregnant women also impacted treatment decisions.
CONCLUSION
By analysing the decision-making process, our result explained the current low uptake rates of antenatal antiviral treatment for HBV among pregnant women. We also concluded that from the health system’s perspective, it was worth providing subsidies for perinatal antiviral treatment to prevent huge expenses generated in the future by chronic HBV complications.
INTRODUCTION
Hepatitis B virus (HBV) infection, as a global health problem, is more likely to occur during infancy and early childhood in developing countries with high prevalence. Mother-to-child transmission (MTCT) usually accounts for almost half of all transmission routes for chronic HBV infections.(1)
The World Health Organization recommends screening for hepatitis B surface antigen (HBsAg) in all pregnant women and the administration of a birth dose of hepatitis B vaccine. The United States Centers for Disease Control and Prevention recommends an additional dose of passive vaccination with hepatitis B immune globulin (HBIG) for infants born to HBsAg-positive pregnant women. Subsequently, infants receive two or three doses of hepatitis B vaccination in the following six months. Despite the relatively excellent efficacy of the HBIG and HBV vaccination, immunoprophylaxis failure may still occur in some cases.(1) In Thailand, the risk of MTCT of HBV has been estimated to be 12% for pregnant women with high hepatitis B viral load.(2) In Australia, transmission rates are 7% from hepatitis B envelope antigen (HBeAg)-positive pregnant women and 9% from pregnant women with very high HBV DNA levels (> 108 copies/mL).(3) In Taiwan and Korea, the immunoprophylaxis failure rate varies from 1% to 11.8%.(4)
From 2011 to 2012, the Health Sciences Authority of Singapore reported at least 21 cases of immunoprophylaxis failure. These infants were born to HBV-carrier pregnant women despite receiving one dose of HBIG at birth and completing the full course of the HBV vaccine.(5) More recently, local data from our group reported a failure rate of 6.0% and 3.0% in the high and low viral load categories, respectively.(6)
A few randomised controlled trials have established that it is safe and effective to provide antiviral therapy, such as lamivudine, tenofovir, and telbivudine, to pregnant women with a high viral load in the third trimester.(7-9) The short-course treatment consistently reduces maternal viraemia and vertical transmission to infants. Before 2012, HBV antiviral treatment during the antenatal period was not widely recommended. However, recent guidelines from the European Association for the Study of the Liver(10) and American Association for the Study of Liver Diseases(11) have advocated treating pregnant women with HBV DNA > 200,000 IU/mL to reduce the rates of MTCT. However, the cost-effectiveness of such treatments to prevent MTCT has not been addressed in Singapore. In the last five years, it was estimated that less than 15% of pregnant women with high viral load (> 200,000 IU/mL) in Singapore agreed to the treatment.(12) By analysing the decision-making process among carrier pregnant women, we aimed to determine, from personal and health system perspectives, if antenatal antiviral treatment is worth taking up. These findings may also provide guidance for healthcare subsidies that may be provided to determine the consumer price of antiviral drugs.
METHODS
A decision tree(13) was constructed to help us to better understand and analyse pregnant women’s considerations on taking antiviral treatment for HBV. We assumed that there was no adverse pharmacological effect for the mother and fetus, and that all infants received the recommended HBIG as well as three doses of HBV vaccine after birth. Once the child was found to be a HBV carrier, the mother would be responsible for any cost incurred due to the infection.
As illustrated in
Fig. 1
Flowchart shows the decision tree for pregnant women who are high viral load carriers in Singapore. Cm: treatment cost (in SGD) for pregnant women to take antiviral therapy during their third trimester; HBV: hepatitis B virus; MTCT: mother-to-child transmission; Pa1: immunoprophylaxis success rate for pregnant women who take antenatal antiviral treatment, or probability that infant may not be infected by HBV; Pa2: probability of vertical transmission for pregnant women who take antenatal antiviral treatment; Pb1: immunoprophylaxis success rate for pregnant women who do not take antenatal antiviral treatment, or probability that infant may not be infected by HBV; Pb2: probability of vertical transmission for pregnant women who do not take antenatal antiviral treatment; X: medical care expenses (in SGD) for chronic HBV infection and related complications
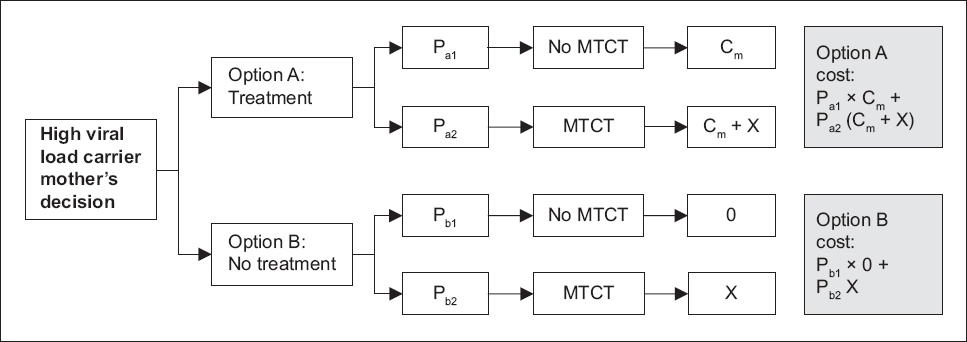
We evaluated the costs and benefits of these two options. We assumed that pregnant women accepted antiviral treatment only when the cost of Option A was lower than that of Option B. The generalised formula (i.e. Formula 1) to arrive at the cost of antiviral treatment was:
Cm = (Pa2 – Pb2) X
In Formula 1, the decision on whether to take treatment was made based on two factors. The first factor was how the probability of MTCT could be reduced by taking treatment (i.e. Pa2 – Pb2). If the drug could effectively reduce the risk of vertical transmission, it was worth taking the treatment; conversely, if taking the drug only brought about minor improvement, the treatment might not be necessary. The second factor was the cost of regular care, or X, for the child as an HBV carrier. If MTCT were to generate a huge financial burden for the woman, she would likely be more willing to invest additional money to prevent it from occurring. When the cost of Option A was equal to that of Option B, the two options were likely indifferent. On the other hand, should the cost of Option A be lower than that of Option B, Option A would be the better choice.
RESULTS
The antiviral medications approved for HBV treatment included lamivudine, entecavir, telbivudine, and tenofovir.
Table I
FDA-approved treatments for chronic hepatitis B virus and cost of proposed treatment.
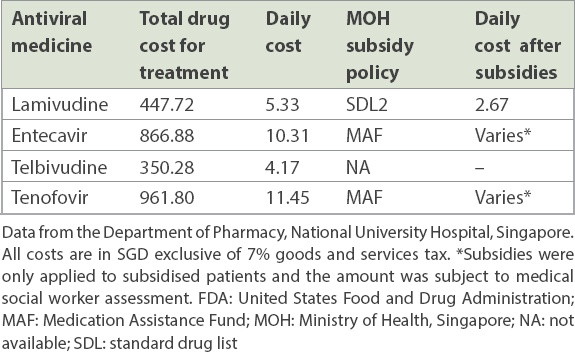
According to a previous study from Singapore,(6) for HBV-carrier pregnant women with high viral load, the immunoprophylaxis success rate (Pb1) was approximately 96.8% without treatment, which was further enhanced to 99.9% (Pa1) following treatment. In other words, pregnant women decided whether it was worth spending a minimum of SGD 350.28 on telbivudine to reduce their child’s HBV infection rate by 3.1%.
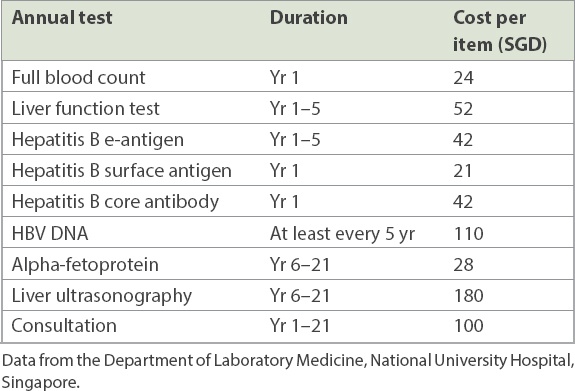
From the pregnant woman’s perspective, if the infant is found to be a carrier, she would be responsible for providing regular care to the infant up to the age of 21 years. Since the likelihood of hepatitis B treatment in the paediatric age group was negligible, the expense of regular care included an annual liver function test and other laboratory tests, such as HBeAg, HBsAg and HBV DNA. The expense, X, was estimated as SGD 7,097.00 in total (
Table II
Price of regular medical care for infants who are hepatitis B virus (HBV) carriers, up to age 21 years.
According to Formula 1, Cm was SGD 220.00. Thus, from an individual pregnant woman’s perspective, the treatment would be cost-effective only when the price was not higher than SGD 220.00 (or SGD 2.62 per day during a 12-week treatment period). In Singapore, the lowest treatment cost was for telbivudine at SGD 350.28, which was more than the expenses incurred otherwise. Therefore, treatment is likely to be unattractive from the pregnant woman’s perspective.
However, from a national perspective, the potential future healthcare costs are huge. HBV, as a chronic disease, remains largely asymptomatic during the early childhood stage. But the risk of HBV-associated liver disease increases as the child reaches adulthood, as does the potential financial burden. The costs of consequent hepatocellular carcinoma treatment and liver transplantation often form the largest financial burden to the family and society. Thus, in a child’s lifetime, the actual regular care expense (X’) would be much higher than an individual’s X costs (year 1–21).
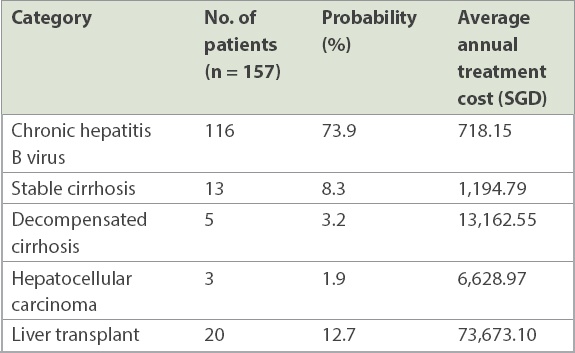
A previous study involving 157 patients with HBV attempted to estimate the average annual treatment cost for different categories of patients in Singapore over five years from the year 2002.(14) We used data from the previous study with calculations, including the probability of each of the potential complications, as well as five years of the screening tests required, to estimate the average annual treatment cost for different patient categories (
Table III
Estimated average annual treatment cost of different patient categories.(14)
According to Formula 1, the cost-effectiveness price of antiviral treatment during the perinatal period was SGD 28.20 per day. Extrapolating these expenses to the future, all current drugs listed earlier cost much less, and thus it becomes worthwhile to take the antiviral treatment in the long run. However, the individual pregnant woman may only evaluate the pros and cons of treatment during the first 21 years of her child’s life, and might not be concerned about possible lifetime adverse consequences or their impact as a public health problem. This potentially leaves the national healthcare system to bear the economic burden.
DISCUSSION
In September 2017, the Ministry of Health, Singapore, published the latest standard drug list to improve the affordability of medications. The addition of lamivudine to the standard drug list(16) gave subsidised patients a 50% subsidy for it. Accordingly, their medication cost would decrease to SGD 2.67 per day, which was close to the pregnant women’s cost-effectiveness price of SGD 2.62.
With this medication subsidy, the drug price was likely to become acceptable to pregnant women, who may then agree to take antiviral treatment. From the perspective of the healthcare system, an investment of SGD 2.67 per day on antiviral treatment during a 12-week period (or SGD 224.28 per person in total) would likely reduce the rate of perinatal transmission of HBV by 3.1%. While the consequent reduction in vertical transmission rates appears small, the measure would encourage more pregnant women to accept antenatal antiviral treatment and thereby prevent huge expenses that would otherwise be incurred and paid for by the healthcare system due to chronic HBV infection and associated complications in the future.
More recently, entecavir and tenofovir have been added to the list of Medication Assistance Fund drugs in Singapore. Patients may also receive subsidies for these two drugs, although the amount varies individually and is subject to means testing by medical social workers.(16) In a situation where the pregnant women’s subjective cognition (ρ) influences the personal decision, Formula 1 could be reformulated to Formula 2 as follows:
Cm = (Pa1 – Pb1) ρX
Pregnant women who have a better understanding of MTCT or are more concerned about the risk may be more sensitive to treatment prices during the antenatal period. For instance, many schools, universities, and companies in China require health checkups for HBV chronic carriage.(17) In such a scenario, ρ > 1 and Cm would be higher. In contrast, other pregnant women may be reluctant to receive treatment if they are unaware of the long-term health consequences of HBV and its impact on their children’s education and future employment opportunities. In such a scenario, ρ < 1, and the treatment would be attractive to them only if Cm was very low. In conclusion, our analysis suggests that from the pregnant woman’s perspective, antenatal antiviral therapy to reduce MTCT was not cost-effective unless healthcare subsidies apply.
In general, the reasons for pregnant women not choosing antenatal antiviral treatment may include: (a) treatment cost (Cm) being too expensive; (b) the difference between the probabilities of MTCT with and without treatment (Pa2 – Pb2) being low; and/or (c) regular care expenses (X) being affordable. Given the high vaccine efficacy and low immunoprophylaxis failure rate, antiviral treatment was unattractive, as the reduction in the probability of MTCT due to added antenatal treatment was not significant. Pregnant women may be more inclined to take it up should the antiviral treatment cost become less than SGD 2.62 per day. This is probably a partial explanation for the current low uptake rate of antenatal antiviral treatment for HBV. Reducing the price of drugs and providing more healthcare subsidies could potentially increase the maternal uptake of such treatment, thus helping to reduce vertical transmission of HBV. While the new subsidy policy has been implemented from September 2017 and will apply to lamivudine, entecavir and tenofovir, its effects on maternal uptake of antenatal antiviral treatment remain to be seen.
Our study had some limitations. First, we only reported vertical transmission rates of MTCT for children born at National University Hospital, Singapore, but nationwide MTCT rates may have been higher. Second, expenses that would be incurred due to chronic HBV infection by MTCT transmission may have been underestimated in our study, as other ancillary costs were not taken into account, such as patient transportation costs to and from medical facilities, time taken off work, drug price inflation in the long term, and emotional costs due to anxiety and stress.
To conclude, in the present scenario, antenatal treatment to prevent HBV vertical transmission is as yet unattractive for pregnant women with high viral load, as the consequent reduction in MTCT rates appears to be small. However, the associated long-term expenses for the healthcare system and the impact of chronic HBV on the family and society at large are considerable. Given that pregnant women may be more inclined to take up antenatal antiviral treatment for HBV if treatment becomes more affordable, it would be helpful to have studies that provide further guidance on healthcare subsidies that determine the consumer price of antiviral treatment drugs. A comprehensive survey of the concerns and perceptions of HBV-carrier pregnant women is also warranted to better understand and manage the various factors at play during the decision-making process.
ACKNOWLEDGEMENTS
We would like to thank Dr Lim Sok Hoon, Senior Principal Pharmacist, National University Hospital, Singapore, for providing the price list of various drugs available at the hospital. We are also grateful to Dr Rajgor Dimple, Department of Paediatrics, National University of Singapore, for her help with editing and revising the manuscript.


