INTRODUCTION
Cross-sectional imaging of the small bowel is replacing conventional barium studies in many centres, with magnetic resonance (MR) enterography becoming particularly popular for the assessment of small bowel Crohn’s disease, given its superior tissue contrast with improved visualisation of the entire bowel and lack of ionising radiation. In addition, MR enterography provides functional information on the peristalsis and distensibility of the involved small bowel segment with dynamic sequences, which is an advantage over computed tomography (CT).
Over 15 years of performing MR enterography examinations, we have encountered a variety of clinically significant findings that mimic or are associated with Crohn’s disease, where the initial clinical suspicion was for Crohn’s disease. As a experience with MR enterography increases, there is also increasing clinician confidence in MR enterography, which has led to referral for evaluation of small bowel diseases other than Crohn’s disease.(1) The manifestations of Crohn’s disease on MR enterography have been well described, but there is comparatively little in the literature about MR enterography appearances of diseases that can mimic Crohn’s disease or diseases other than Crohn’s disease.(2)
In this pictorial essay, we present a range of enteric and extra-enteric diseases that mimic or are associated with Crohn’s disease, and provide examples of imaging features. In addition, we review the use of MR enterography for the evaluation of small bowel diseases other than Crohn’s disease. Our aim is to raise awareness of the range of diseases that may be encountered on MR enterography, and to add to the limited literature on findings unrelated to Crohn’s disease.
MR enterography technique
Our MR enterography technique employs a biphasic oral bowel distending agent, prepared by forming a solution containing 0.2% locust bean gum and 2.5% mannitol to produce consistently satisfactory small bowel distension. Patients drink up to 1.5 L of agent over 45 minutes prior to scanning. After planning using scout acquisitions with the patient in supine position, we administer an intravenous spasmolytic agent to minimise motion artefacts related to peristalsis, usually 20 mg hyoscine-N-butylbromide, with 0.5-mg glucagon as an alternative. Only breath-hold acquisition sequences are employed. In all cases, we acquire T2-weighted half-Fourier acquisition single-shot turbo spin-echo (HASTE) and true fast imaging with steady state precession (FISP) sequences in the coronal and axial planes. Fat saturation may be applied to increase the conspicuity of inflammatory changes in the bowel wall and perienteric tissues. Diffusion-weighted imaging is acquired when active inflammatory disease is suspected. In cases where contrast enhancement characteristics may aid lesion detection and/or characterisation, we also acquire T1-weighted volumetric interpolated breath-hold examination (VIBE) sequences in the axial and coronal planes, before and after administration of intravenous gadolinium chelate agents at 0.1 mg/kg.
Imaging findings
This pictorial essay is presented in three sections. The first section describes enteric diseases that can mimic the presentation of Crohn’s disease. These cases were encountered when Crohn’s disease was initially suspected by clinicians. The second section describes extra-enteric findings that mimic the presentation of Crohn’s disease or are associated with Crohn’s disease. The third section describes a range of rare enteric diseases unrelated to Crohn’s disease but which have been referred for MR enterography over the years.
SECTION 1: ENTERIC DISEASES THAT MAY MIMIC CROHN’S DISEASE
Superior mesenteric artery syndrome
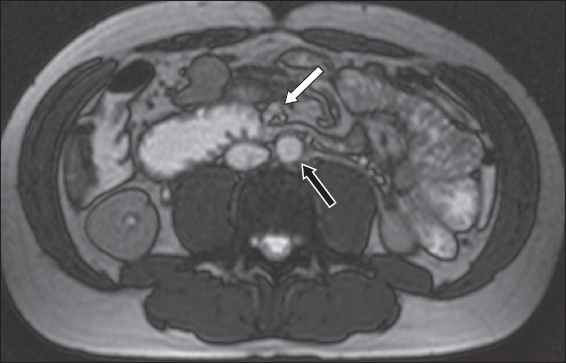
Superior mesenteric artery syndrome is a potentially life-threatening disease characterised by compression of the third part of the duodenum between the abdominal aorta and the superior mesenteric artery (SMA) (
Fig. 1
A 31-year-old man had a six-year history of abdominal symptoms and clinical suspicion for small bowel Crohn’s disease. He had lost some weight but was not cachectic. Axial true fast imaging with steady state precession (FISP) MR enterography image shows bowel dilatation upstream from focal extrinsic compression across the third part of the duodenum, resulting from an unusually narrow angle between the superior mesenteric artery (arrow) and the aorta (black arrow).
Intussusception
Intussusception is a telescopic invagination of the bowel into the lumen of an adjacent bowel segment. It is a common cause of small bowel obstruction in children but is less common in adults. In adults, almost 90% of intussusceptions have a pathological lead point (usually neoplastic).(4) Some patients may present acutely and some with symptoms of intermittent bowel obstruction over several weeks, which may lead to clinical suspicion for inflammatory bowel disease.
A target-like (bowel-into-bowel) appearance of the bowel is pathognomonic. On transverse section, the inner central structure is the invaginating loop (intussusceptum), which is enveloped by the receiving loop (intussuscipiens) (
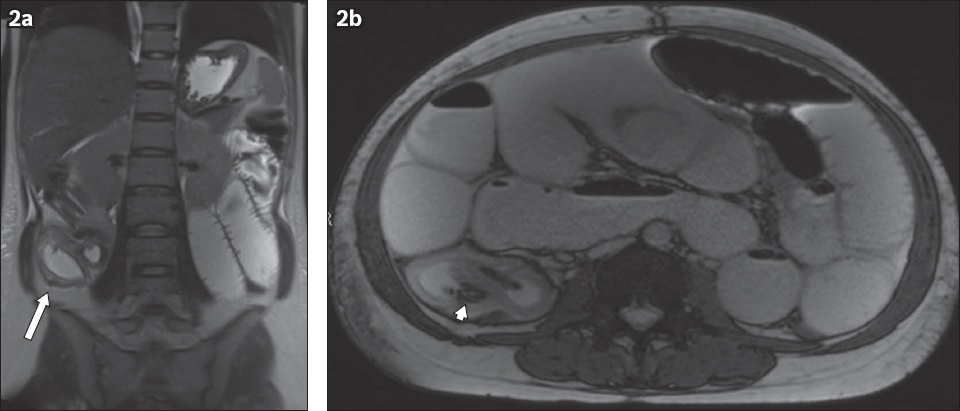
Fig. 2
A 30-year-old patient had a short history of abdominal pain and vomiting. (a) Coronal half-Fourier acquisition single-shot turbo spin-echo (HASTE) and (b) axial true FISP MR enterography images show generalised dilatation of fluid-filled small bowel loops associated with a focal transition point in the right lower quadrant, where a double bowel wall appearance is evident (arrow). The transition point presents a bowel-into-bowel appearance suggesting ileocolic intussusception. Curved linear low-signal bands (arrowhead) within the intussusceptum represent the ‘Indian ink’ chemical shift artefact between mesenteric fat and the surrounding intraluminal fluid. The findings were confirmed during surgery and a Meckel’s diverticulum was identified as the lead point.
Adhesion
Abdominal adhesions are fibrous bands connecting loops of bowel to each other or the peritoneum. They form in response to surgical handling or due to inflammatory intra-abdominal disease, and cause bowel obstruction either by direct extrinsic compression or by acting as a pivot for torsion or kinking of the bowel (
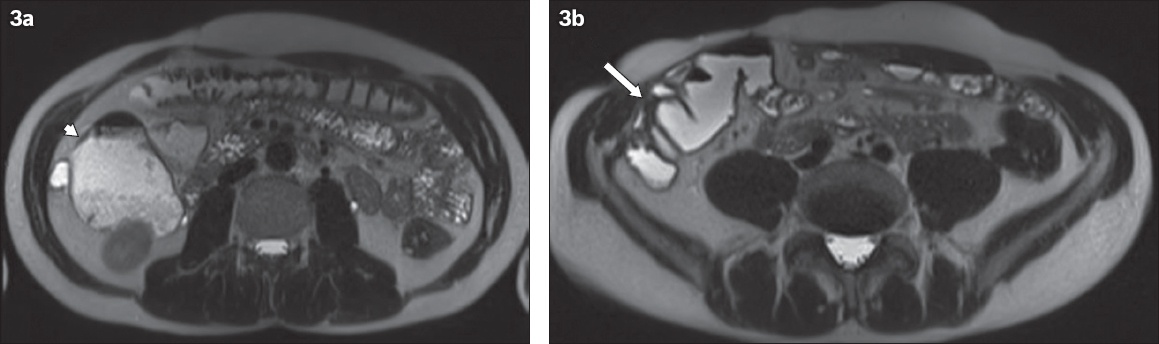
Fig. 3
A 49-year-old man had a history of previous right hemicolectomy for intussusception as a child and presented with an acute abdomen. (a & b) Axial HASTE MR enterography images show dilated small bowel loops in the right quadrant (arrowhead) associated with a transition point (arrow), at which there is a cluster of tethered, angulated small bowel loops adjacent to the abdominal wall. The diagnosis was post-surgical adhesion related to the previous surgical wound.
Malrotation
A wide range of congenital gastrointestinal anomalies may be encountered, including luminal stenosis, anomalies of rotation or fixation, mesenteric cysts, anorectal anomalies and intestinal duplication. Patients may be asymptomatic and these anomalies may be discovered incidentally, or they may present with intermittent abdominal symptoms suggestive of Crohn’s disease or intermittent bowel obstruction.
Intestinal malrotation occurs when the midgut fails to complete its 180° counterclockwise rotation in embryo. The duodenojejunal junction lies low and to the right of the midline, with the small bowel lying in the right and the mid abdomen (
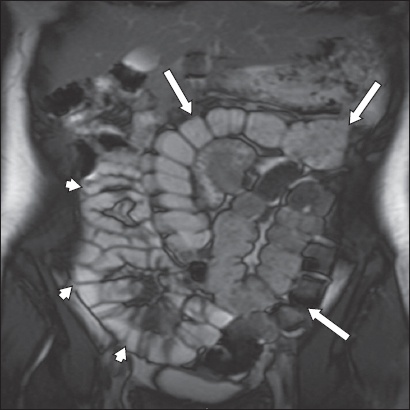
Fig. 4
A 19-year-old woman presented with intermittent abdominal pain and clinical suspicion for Crohn’s disease. Coronal true FISP MR enterography image shows malrotation with the entire small bowel in the right side of the abdomen (arrowheads) and the entire large bowel in the left (arrows). There was no evidence of Crohn’s disease.
SECTION 2: EXRA-ENTERIC DISEASES THAT MAY MIMIC OR BE ASSOCIATED WITH CROHN’S DISEASE
Urinary tract
Common diseases of the urinary tract (obstruction, inflammation, neoplasm) often present with non-specific abdominal symptoms that can mimic bowel pathology, including pain, nausea, vomiting or palpable mass. Urological complications are common in patients with Crohn’s disease and may be related to the disease itself or to its treatment.(7) Affected patients may have bowel symptoms that overshadow the urinary tract disease, resulting in delayed diagnosis. Two illustrative examples were encountered in patients with known or suspected Crohn’s disease.
Hydronephrosis
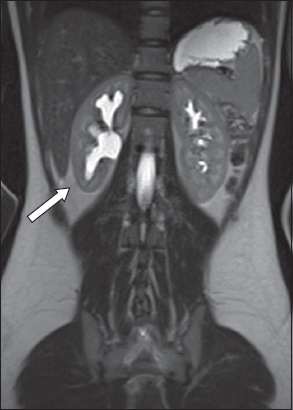
Hydronephrosis may be either obstructive or non-obstructive. Non-obstructive hydronephrosis is usually due to congenital structural abnormality, such as vesicoureteral reflux (
Fig. 5
A 16-year-old girl had intermittent abdominal pain, poor appetite, weight loss and clinical suspicion for Crohn’s disease. Coronal HASTE MR enterography image shows unilateral right hydronephrosis without perinephric inflammatory change (arrow). A subsequent radioisotope excretion renogram (not shown) confirmed the impression of non-obstructive hydronephrosis. It transpired that there was a previous history of vesicoureteral reflux as a young child. No active Crohn’s disease was identified on MR enterography.
Renal cell carcinoma
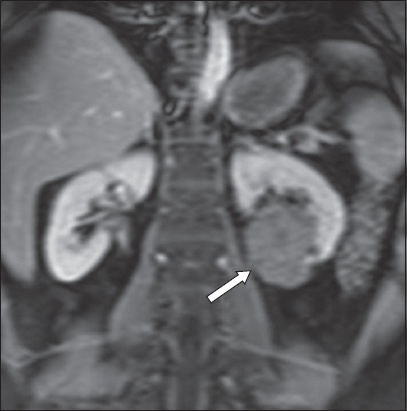
Around 50% of renal cell carcinomas (RCCs) are identified incidentally on cross-sectional imaging investigation, as they are usually asymptomatic until very large or metastasised (
Fig. 6
A 59-year-old woman with a history of Crohn’s disease presented with abdominal pain and altered bowel habit. Coronal gadolinium-enhanced VIBE MR enterography image shows the incidental finding of a 5-cm round, poorly enhancing mass arising from the lower pole of the left kidney (arrow). Histology of the surgical resection specimen confirmed the diagnosis of mixed papillary and clear cell carcinoma.
Gynaecological
Most female patients referred for MR enterography are of reproductive age, so presentations with iliac fossa or pelvic symptoms may be due to a gynaecological pathology. In these patients, ovarian cysts (and their complications) and endometriosis are not uncommon and may be identified on MR enterography in cases where Crohn’s disease has been suspected.
Endometriosis/haemorrhagic ovarian cyst
Patients with endometriosis (
Fig. 7
A woman of child-bearing age presented with a history of bowel dysfunction and anaemia. (a) Axial true FISP and (b) coronal HASTE MR enterography images show normal appearance of the small bowel, but examination revealed a few cystic lesions in the pelvis compatible with endometriotic cysts. The right adnexal cystic lesion contains a fluid-fluid level (arrowhead). The 7-cm left adnexal cystic lesion (arrows) shows relative decreases in T2-W signal intensity or ‘T2 shading’.
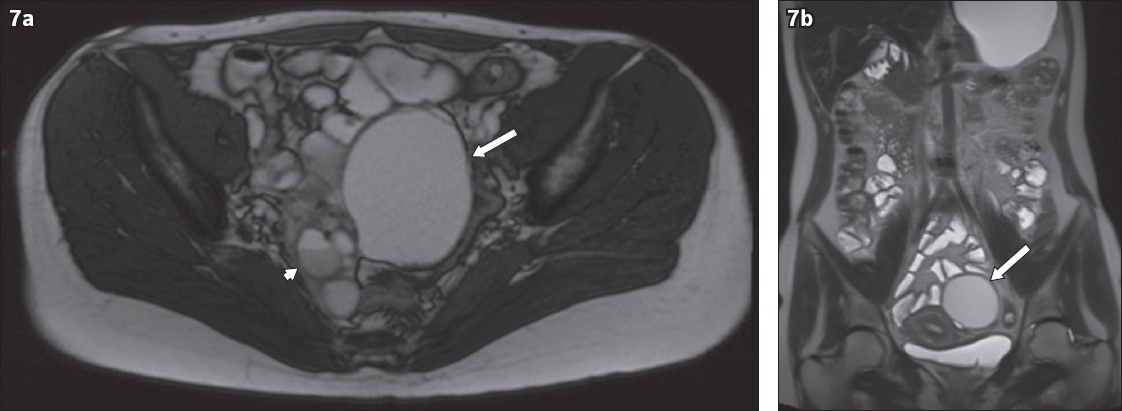
Fig. 8
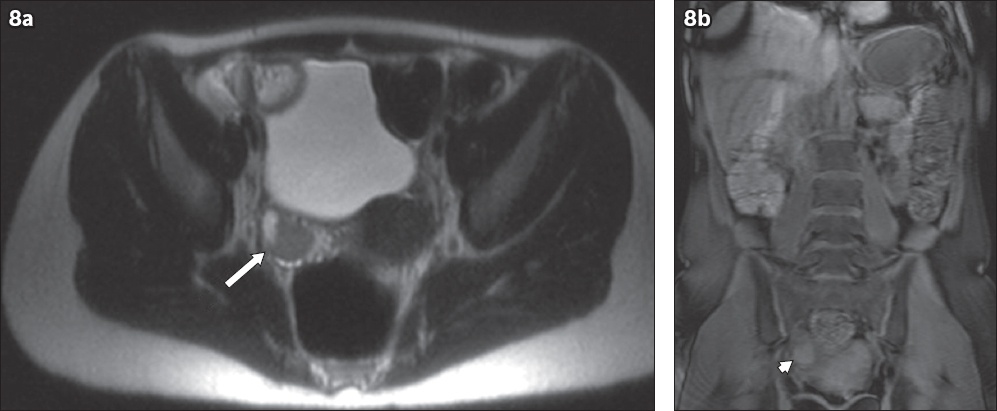
A 16-year-old girl had a short history of right iliac fossa pain. (a) Axial HASTE MR enterography image shows a normal small bowel but an abnormal right ovary with mixed high and low signal intensity on T2-W HASTE images (arrow). (b) Coronal unenhanced T1-W volumetric interpolated breath-hold examination (VIBE) MR enterography image shows intermediate signal intensity (arrowhead). The MR enterography findings suggest a diagnosis of recent right ovarian haemorrhage. Follow-up US imaging six months later was normal with complete resolution of the haemorrhagic cyst.
Retroperitoneal
The retroperitoneum may harbour a wide range of inflammatory and neoplastic conditions, but it is difficult to evaluate clinically. Consequently, retroperitoneal pathology may be identified late after a protracted non-specific presentation.
Pheochromocytoma/paraganglioma
Pheochromocytomas are chromaffin cell tumours arising in the adrenal medulla. Paragangliomas arise from extra-adrenal chromaffin cells of the sympathetic nervous system (usually the retroperitoneum) or parasympathetic ganglia (usually adjacent to the aortic arch, in the neck or skull base). Hypertension and other catecholamine-related symptoms occur in approximately 90% of the population.(12) Gastrointestinal presentations are common and include abdominal pain, vomiting and nausea, which can mimic Crohn’s disease. On MR imaging, the tumours are characteristically markedly hyperintense on T2-weighted imaging and slightly T1 hypointense in relation to the remainder of the adrenal (
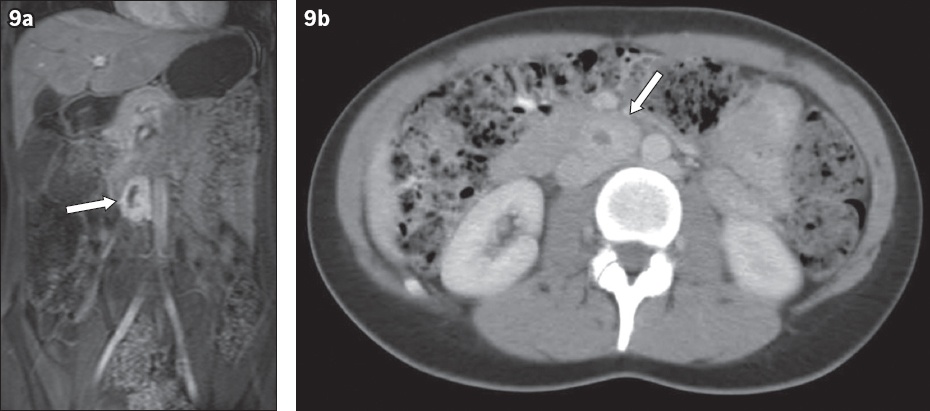
Fig. 9
A 27-year-old woman had a history of upper abdominal pain and intermittent sweating episodes. (a) Gadolinium-enhanced coronal VIBE MR enterography image shows a normal duodenum, but there is an ovoid soft tissue mass (arrow) to the right of the aorta with avid enhancement of a thick wall and a non-enhancing centre. (b) Axial coronal CT image done previously shows duodenal wall thickening (arrow), and Crohn’s disease was suspected. Biochemical findings reflected high levels of urinary catecholamine excretion. Histology of the resected surgical specimen confirmed a diagnosis of metabolically active paraganglioma.
Abdominal wall
Abdominal wall abnormalities may mimic Crohn’s disease or be identified incidentally when reviewing MR enterography. The proximity of the small bowel to the abdominal wall predisposes the bowel to be involved in abdominal wall pathologies and vice versa.
Abdominal wall hernia
Abdominal wall hernia is a defect in the muscular wall of the abdomen through which the bowel, mesentery or omentum may protrude. Clinical manifestations may be non-specific and range from mild intermittent discomfort with reducible hernias to severe acute abdominal crisis if the bowel is incarcerated or strangulated. Incisional hernias are a result of an incompletely healed surgical incision. Parastomal hernias occur through the defect formed when creating a stoma (
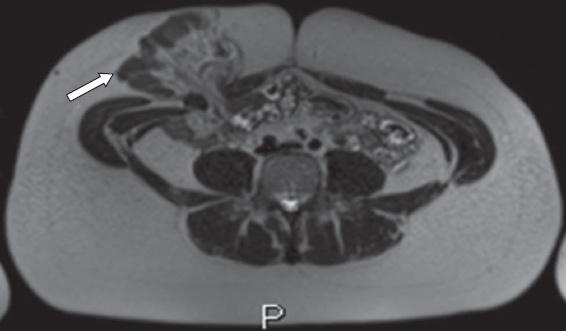
Fig. 10
A 15-year-old boy had a history of previous subtotal colectomy for indeterminate colitis and presented with a history suggestive of intermittent bowel obstruction, with vomiting and decreased stoma output. Axial HASTE MR enterography image shows several loops of small bowel (arrow) herniated through the stoma site. As the neck of the abdominal wall defect is wide, the symptoms were attributed to intermittent torsion of the herniated small bowel loops. No active Crohn’s disease was identified on MR enterography.
SECTION 3: ENTERIC DISEASES OTHER THAN CROHN’S DISEASE REFERRED FOR MR ENTEROGRAPHY
As experience and confidence accumulates, we have found that more clinicians are keen to refer patients with more unusual small bowel disease for MR enterography. Typically, these are cases where the conventional practice would be to perform barium studies or CT examinations. MR enterography may be preferred because, like the Crohn’s disease population, patients may be young and require repeated imaging.
Coeliac disease
Coeliac disease is an autoimmune disorder associated with a genetic predisposition. Patients typically present with weight loss with features of malabsorption, including steatorrhoea. Hypersensitivity to the insoluble protein gluten results in flattening of the small bowel mucosa with truncation of the villi. Mural findings are similar to the classic barium appearances, including abnormalities of the fold pattern, such as reversal of the jejunoileal fold pattern, with decreased number of folds per inch in the jejunum and an increased number of folds per inch in the ileum. This finding is specific to coeliac disease (
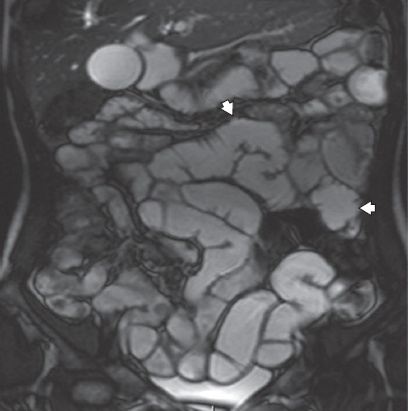
Fig. 11
A 51-year-old woman with longstanding coeliac disease was examined for possible small bowel lymphoma. Coronal HASTE MR enterography image shows capaciousness and relative baldness (‘ilealisation’) of the jejunum (arrowheads), which is characteristic of incompletely controlled coeliac disease. There was no evidence of lymphomatous transformation.
Systemic sclerosis
Systemic sclerosis is characterised by widespread collagen deposition in the skin, blood vessels, muscles and internal organs. Up to 50% of cases include small bowel involvement. The classical small bowel follow-through finding is a ‘hide-bound’ appearance of the jejunum and proximal ileum caused by crowding of the valvulae. Hypomotility leads to stasis, bowel dilatation and pseudo-obstruction (
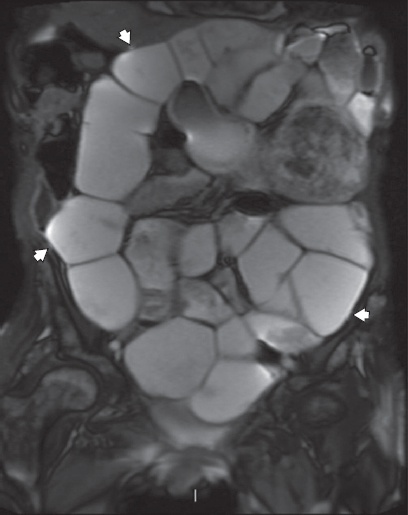
Fig. 12
A 72-year-old woman with systemic sclerosis presented with a two-year history of weight loss and diarrhoea. Coronal true FISP MR enterography image shows gross generalised small bowel dilatation with a paucity of valvulae and no discrete transition point (arrowheads). The findings are typical of pseudo-obstruction associated with systemic sclerosis.
Peutz-Jeghers syndrome
Autosomal dominant Peutz-Jeghers syndrome combines mucocutaneous pigmentation and hamartomatous gastrointestinal polyposis. Hamartomatous polyps result from proliferation of all the three layers of the mucosa and may occur anywhere, with the small bowel being the commonest site. The malignant potential of the polyps is estimated to be 2%-20%.(13) Differentiation from other small bowel polyposis syndromes such as familial adenomatous polyposis, Cronkhite-Canada syndrome and juvenile polyposis requires correlation with clinical and histopathological findings. MR enterography is performed to monitor for polyps exceeding 1.5-2 cm in size, which are considered for resection in view of their malignant potential. Polyps appear as luminal filling defects, often with visible stalks, which enhance homogeneously. They may cause intussusception (
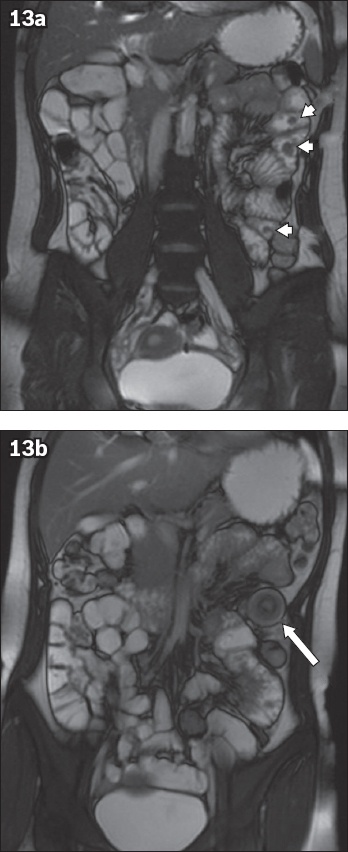
Fig. 13
A 22-year-old woman with Peutz-Jeghers syndrome had a recent history of intermittent abdominal pain. Coronal true FISP MR enterography images show (a) multiple small polyps (arrowheads) in the jejunum and (b) a transient intussusception (arrow) in this region, presumably caused by one of the polyps acting as a lead point. Transient intussusception is a recognised complication of small bowel polyposis associated with Peutz-Jeghers syndrome.
Primary adenocarcinoma of the small bowel
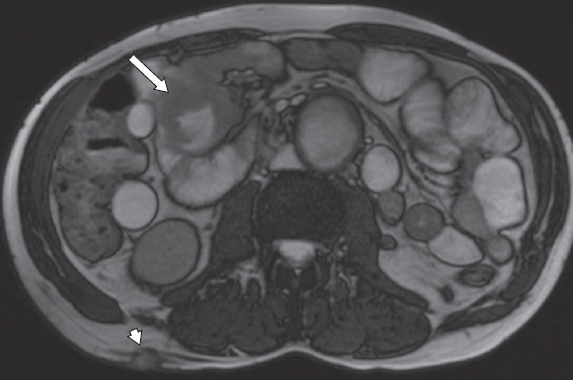
Adenocarcinoma is the commonest of the (rare) malignant primary small bowel neoplasms.(14) They are usually seen in the duodenum and proximal jejunum, and rarely in the distal ileum. Typically, they are heterogeneous, enhancing, eccentric solitary masses, although they may ulcerate or manifest as irregular circumferential wall thickening (
Fig. 14
A 55-year-old man with a long history of poorly controlled coeliac disease underwent investigations for suspected lymphomatous transformation. Axial true FISP MR enterography image shows focal irregular wall thickening in the ileum (arrow) associated with mild, generalised distension of the upstream small bowel. There are multiple subcutaneous nodules (arrowhead) compatible with subcutaneous metastasis. The presumed diagnosis was lymphoma, but biopsy revealed a diagnosis of primary adenocarcinoma of the small bowel.
Lymphoma
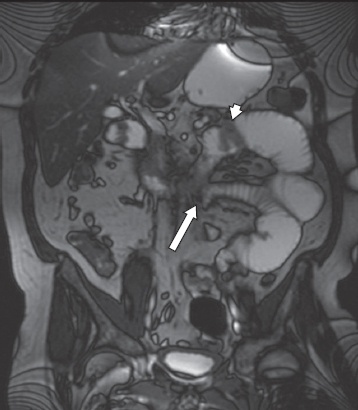
The terminal ileum is the commonest site for lymphomas. Most are non-Hodgkin’s lymphomas and have a wide range of appearances. Typically, there is circumferential wall thickening and fold effacement, accompanied by mesenteric lymphadenopathy, all due to lymphomatous infiltration. Infiltration that reduces the integrity of the muscularis layer may result in aneurysmal dilatation of the bowel lumen. Polypoid protuberances may act as lead points, causing intussusception. Deeper transmural infiltration may lead to perforation and fistula formation, which may be difficult to distinguish from Crohn’s disease (
Fig. 15
A 52-year-old man with Langerhans cell histiocytosis presented with persistent vomiting and food aversion. Upper gastrointestinal endoscopy biopsy revealed cytomegalovirus duodenitis, and a barium follow through demonstrated proximal bowel dilatation with hold-up of barium in the mid jejunum. Coronal true FISP MR enterography image shows focal nodular wall thickening in the fourth part of the duodenum (arrowhead) and a transition point (without any focal bulk disease) in the jejunum at the point where the jejunum was adherent to the third part of the duodenum (arrow). Histology of the surgical resection specimen revealed non-Hodgkin’s lymphoma at both sites.
CONCLUSION
This pictorial review familiarises radiologists with the MR enterography appearance of a range of diseases unrelated to Crohn’s disease, with and without bowel wall involvement, which may clinically mimic Crohn’s disease. In addition, we included a range of unusual enteric conditions unrelated to Crohn’s disease that clinicians have referred for MR enterography over the years as experience and confidence accumulates.
SMJ-62-181.pdf


