Abstract
INTRODUCTION
Pulmonary arterial hypertension (PAH) is associated with high medical and pharmaceutical costs. Phosphodiesterase type 5 (PDE5) inhibitors have been found to be beneficial but costly. They are not subsidised in Singapore except via the Medication Assistance Fund (MAF) Plus scheme. In this study, we described the help-seeking behaviour of patients and funding strategies for Singaporean patients on PDE5 inhibitors in our registry.
METHODS
We consecutively recruited all patients with PAH who presented to our pulmonary hypertension specialty centre between 1 January 2003 and 29 December 2016. Singaporean patients on PDE5 inhibitors were included. Data recorded and analysed for this study included baseline demographics, whether the patients received MAF Plus funding, percentage of funding, and any additional source of subsidies.
RESULTS
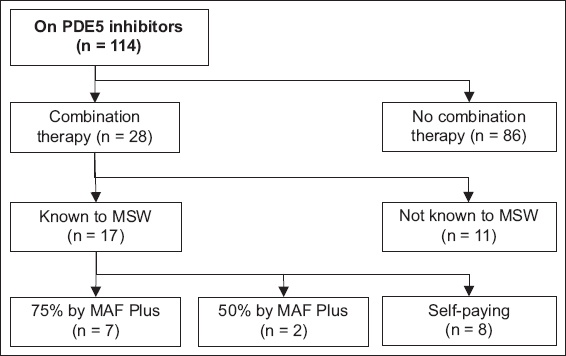
114 (77.0%) of 148 patients in the registry were Singapore citizens on PDE5 inhibitors. 75 (65.8%) of these 114 patients had been seen by a medical social worker, of whom 16 were on MAF Plus funding. 14 of the remaining 59 patients were subsidised by MediFund, whereas the remainder were self-paying. 30 (26.3%) patients in total were on some form of subsidy, and 28 (24.6%) patients were on combination therapy. Of this group, nine were receiving MAF Plus subsidies.
CONCLUSION
Fewer than expected patients were found to be receiving drug subsidies for PAH. This was partly due to insufficient referrals and lack of requests for financial assistance. Patients on combination therapy had greater financial challenges. This study should spur us on to study funding gaps further and address them.
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a rare and fatal disease.(1) It is highly morbid and incurable. Recently, the availability of specific targeted therapies has resulted in improvements in patient outcomes. It has been shown that administering combination treatment at the onset may result in even better results.(2,3) However, the cost of such therapy – which is often lifelong – is high.
Our institution maintains a registry of PAH patients that includes their clinical presentation, treatment and outcomes. Our previously published data shows that the majority of PAH patients in our institution are on phosphodiesterase type 5 (PDE5) inhibitors such as sildenafil and tadalafil.(4) PDE5 inhibitors have pulmonary vasodilatory effects and have been found to improve exercise capacity and haemodynamics in PAH patients.(5-7) As such, they have been endorsed by international societies as a first-line therapeutic agent.(8) The cost of treatment (per patient per month) in our institution is around SGD 500 for sildenafil and SGD 1,000 for tadalafil. PDE5 inhibitors are not on Singapore’s standard drug list (SDL) and do not qualify for Medication Assistance Fund (MAF) subsidies. Patients who are unable to afford these medications are referred to a medical social worker to assess their financial needs, and alternative methods of funding are explored. One option that has helped many patients with the high costs is the MAF Plus scheme, which was put in place for patients on high-cost drugs that are not on the SDL or MAF list.
In this study, we aimed to describe the help-seeking behaviour of and funding strategies for Singaporean patients on PDE5 inhibitors in our registry.
METHODS
We consecutively recruited all patients with PAH who presented to our pulmonary hypertension (PH) specialty centre between 1 January 2003 and 29 December 2016. The diagnosis of PAH was defined as mean pulmonary arterial pressure > 25 mmHg at rest and pulmonary capillary wedge pressure < 15 mmHg on right heart catheterisation, with exclusion of Groups 2, 3, 4 and 5 PH. Patients were enrolled if the clinical evidence was consistent with the accepted definition of PAH according to the managing PH specialist, even if the invasive haemodynamic data was incomplete. All enrolled patients had supportive evidence on echocardiography. Echocardiographic criteria were: (a) pulmonary arterial systolic pressure > 40 mmHg, estimated via tricuspid regurgitation jet; (b) features of right ventricular pressure overload with normal left ventricular systolic and diastolic function; and (c) normal left atrial volume. Patients with more than one contributor of PH were included if PAH was diagnosed to be the primary pathology. We decided on this approach for better generalisability to a real-world setting in which not all patients would have invasive haemodynamic data and often have multifactorial causes of PH. For this analysis, we restricted the study population to only patients on PDE5 inhibitors, as monotherapy or combination therapy. Foreigners and permanent residents were not included, as they are not eligible for local subsidies and are hence not representative of local PAH financing.
Data recorded and analysed for this study included the usage of drug therapy, whether the patients were receiving MAF Plus funding and their percentage of funding if they were, as well as any additional source of subsidies.
RESULTS
Out of a total of 148 patients in the registry, 114 (77.0%) were Singapore citizens on PDE5 inhibitors, either as monotherapy or combination therapy. The mean age was 51.6 years with a 76.3% female predominance. The racial breakdown was consistent with our national census, with 71 (62.3%) Chinese, 30 (26.3%) Malay and 12 (10.5%) Indian patients, as well as 1 (0.9%) from the ‘others’ group. The most common aetiologies were congenital heart disease-associated PAH (CHD-PAH; 36.8%), followed by idiopathic PAH (26.3%) and connective tissue disease-associated PAH (CTD-PAH; 25.4%). Other less common aetiologies were hereditary PAH, portopulmonary PAH and pulmonary veno-occlusive disease. Most cases (59.6%) were prevalent, whereas the remainder were incident.
Most of our patients were symptomatic, the majority being Class II or III (37.7% and 21.1%, respectively) based on the New York Heart Association functional classification. The remaining haemodynamic and prognostic data is presented in
Table I
Baseline characteristics of Singaporean pulmonary arterial hypertension (PAH) patients on phosphodiesterase type 5 inhibitors (n = 114).
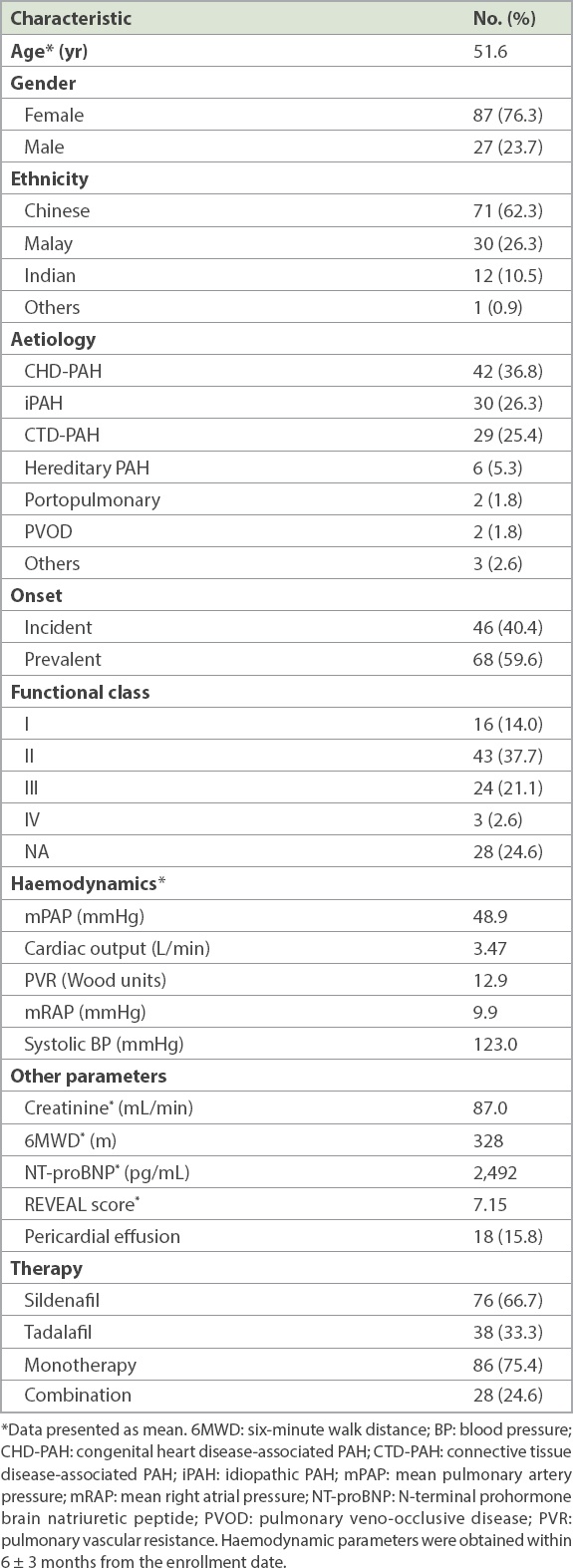
75 (65.8%) patients had been referred to a medical social worker (MSW). 16 (21.3%) of these patients were eligible for MAF Plus funding: 14 were eligible for 75% funding, whereas two were eligible for 50% funding. The remaining drug costs were mainly paid for by the government endowment fund, MediFund, or an institutional endowment fund for heart patients, the Heart Fund. One patient also received partial funding from the Singapore Heart Foundation (SHF). Of the 59 patients who were previously reviewed by an MSW but were not receiving MAF Plus funding, 14 required assistance from MediFund. The remaining 45 patients paid for their own therapy (
Fig. 1
Chart shows breakdown of funding for Singaporean pulmonary arterial hypertension patients on PDE5 inhibitors. MAF: Medication Assistance Fund; MSW: medical social worker; PDE5: phosphodiesterase type 5; SHF: Singapore Heart Foundation
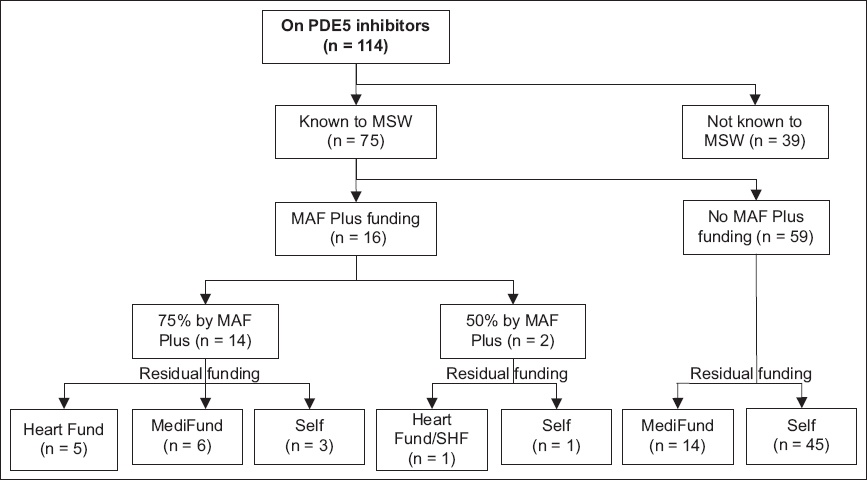
Fig. 2
Chart shows breakdown of funding for Singaporean pulmonary arterial hypertension patients on combination therapy. MAF: Medication Assistance Fund; MSW: medical social worker; PDE5: phosphodiesterase type 5
DISCUSSION
PAH is a highly morbid condition, although recent medical advances have allowed for an expanded therapeutic armamentarium that includes PDE5 inhibitors, endothelin receptor antagonists, soluble guanylyl cyclase stimulators and prostacyclin analogues.(8) The disease and its treatment has been associated with a substantive economic burden on patients and the healthcare system. A systematic review showed that estimated total healthcare costs ranged from USD 2,476 to USD 11,875 per patient per month, with pharmacy costs being an important driver. Sildenafil was reported to be cost-effective and associated with lower costs and higher efficacy.(9)
In our institution, the cost of PDE5 inhibitor therapy is estimated to be about SGD 500–1,000 per patient per month prior to funding. For patients on combination therapy, pharmacy costs are cumulative. This would be a significant burden for most Singaporeans who do not receive subsidies, considering that the median income per household member was SGD 2,500 in 2019.(10)
The high morbidity of PAH leads to high healthcare costs even after initiation of PAH therapy. A study of United States-based insurance claims showed that total healthcare costs did not change after initiating PAH therapy. The cost savings obtained from decreasing non-pharmacy health costs such as hospital admissions were made up for by the increased pharmacy costs of PAH drugs, which were USD 19,961 over a six-month period.(11)
Healthcare financing in Singapore is built on a tiered co-payment model. The first tier is government subsidies. The next is the compulsory medical savings scheme MediSave, which is claimable for hospitalisation as well as selected outpatient chronic condition expenditure. The third tier is provided through a national health insurance scheme, MediShield Life, that covers hospitalisation and selected costly outpatient treatments such as dialysis and chemotherapy or radiotherapy. The last tier of funding is through MediFund, an endowment fund for patients who require additional financial assistance on top of the existing provisions.(12,13) The Ministry of Health provides a blanket subsidy for all drugs on the SDL for patients served at subsidised specialist outpatient clinics and polyclinics, and can be up to 75% of the drug cost. The MAF was set up in 2010 to provide subsidies for newer or high-cost medications that are not on the SDL for specific indications. The SDL and MAF list are reviewed periodically by a drug advisory committee.(14-16)
PDE5 inhibitors are not MediSave claimable and are not on either of the drug subsidy lists. Hence, patients who need financial assistance have to apply for special funding through the MAF Plus scheme. The threshold criteria for qualification is an income per household member of SGD 2,600 or less, approval of the medication by the Health Sciences Authority, and the lack of a cheaper alternative. The MSW plays an integral role in financial assessment and the application process.
Our patients are similar in profile to those of other international registries, although idiopathic PAH is less common locally. They tend to present symptomatically with an intermediate risk profile.(1,4) This is significant, as there are growing international recommendations to initiate treatment with combination therapy for patients who are at intermediate or high risk.(8)
In the present study, only two-thirds of the patients were reviewed by an MSW. Of these patients, 59 (78.7%) eventually did not receive MAF Plus funding. 14 of these patients already had MediFund assistance, which was sufficient to cover drug costs. Of the remaining 45 patients, 29 were assessed by a MSW prior to the inception of the MAF Plus scheme or were not reviewed for the purposes of financial assessment. A minority were not keen to go through the financial assessment process.
It is striking that only 26.3% of all patients on PDE5 inhibitors received at least a partial subsidy. Considering that the median income per household member in 2019 (SGD 2,500) was lower than the threshold for MAF Plus (SGD 2,600), our patients appeared to be utilising less subsidies than expected. In our analysis, we also noticed that one-third of our patients were not referred for MSW assessment for subsidies. Of those who were seen by an MSW but did not receive subsidies, most patients did not request for financial assistance, or were seen in an era in which MAF Plus was not available and were lost to follow-up thereafter.
Although our patients were in the intermediate risk category on average, only one-quarter of them were on combination therapy. 61% of these patients were seen by an MSW and over half of those assessed were on MAF Plus funding – with the majority being in a higher needs category on 75% funding. Given the high costs of PAH drugs, especially with multi-drug usage, the aforementioned costs reflect the additional financial challenges that patients on combination therapy face, and constitute a potential reason for the low uptake of combination therapy.
Furthermore, there is currently no system of routine MSW referrals upon diagnosis of PAH and initiation of therapy; instead, referrals occur when the patient expresses a need. The data presented suggests that routine referrals for financial assessment would be beneficial and could possibly increase the uptake of PAH therapy. Reviews for subsidies should be revisited periodically, especially as policies change.
These findings need to be explored in future studies to determine if there is a lack of awareness of the availability of financial assistance or a cultural reluctance in local patients to request for financial help. We can also further explore whether drug costs are a major obstacle to patient acceptance of PAH therapy.
This study was not without limitations. We were not able to obtain household financial details for individual patients in this study, which would have enabled us to understand if the patients who had not seen MSWs were those who had the financial means to pay on their own. Also, patient numbers were small and from a single academic centre. We were also unable to study clinical outcomes among the patients who received funding compared to those who did not receive funding.
In conclusion, PAH is a disease with significant morbidity. Drug therapy is efficacious but associated with high costs. Although financial help is available, we identified that fewer patients received or accepted funding than expected, likely due to insufficient referrals to MSWs for drug subsidies. Even when they were referred, there was no follow-up request for financial assistance. Patients on combination therapy face additional financial challenges that could be a barrier to greater uptake of more effective therapy. The increasing number of agents with higher cost in the near future is likely to result in a more challenging financing environment, which needs to be planned for as soon as possible.
About the First Author

Dr Lim Yinghao is an Associate Consultant with the Department of Cardiology, National University Heart Centre, Singapore. His clinical interests are in structural heart disease, adult congenital heart disease, pulmonary hypertension and cardiac imaging. He performs structural and congenital heart intervention as well as interventional echocardiography. Additionally, he also performs transoesophageal echocardiography. Dr Lim is an established medical educator and is course co-director for the Chia Boon Lock Cardiology Review Course and Cardiology PACES Course. He is a core faculty member of the Cardiology Senior Residency Programme. His research interests are in pulmonary hypertension, structural heart disease as well as medical innovation.


