Abstract
INTRODUCTION:
Acute aortic dissection (AAD) is a rare and potentially fatal condition that has been known to be missed in diagnoses. Our primary objective was to determine if the availability of 24-hour emergency department (ED) specialist coverage and an on-site computed tomography (CT) scanner reduced the rate of missed diagnoses of AAD.
METHODS:
We selected records of patients diagnosed with dissection of the aorta from a hospital’s discharge database and death register in the period of January 1998 to December 2014. AAD was defined as missed if imaging to diagnose AAD or a cardiology/cardiothoracic surgical consultation was not obtained in the ED. We compared the rates of missed diagnosis before and after the availability of 24-hour ED specialist coverage and an on-site CT scanner in the ED.
RESULTS:
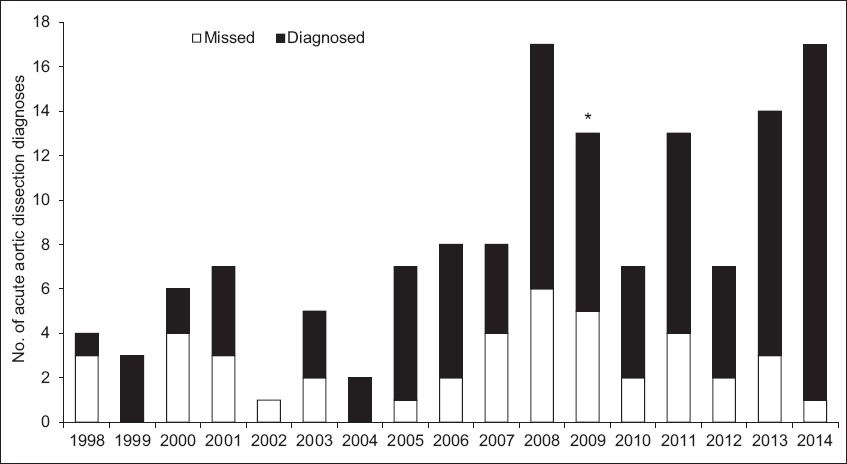
Among 145 patients, 42 (29.0%) had a missed diagnosis. The proportion of missed AAD was lower in the post-implementation period compared to the pre-implementation period (20.0% vs. 37.3%, odds ratio [OR] 0.42, 95% confidence interval [CI] 0.20‒0.89; p = 0.023). After adjusting for confounders, the difference remained significant (OR 0.31, 95% CI 0.14‒0.70; p = 0.005). In the post-implementation period, concurrent signs of congestive cardiac failure (OR 33.51, 95% CI 1.42‒789.20; p = 0.024) and absence of a widened mediastinum on chest radiography (OR 11.52, 95% CI 1.37‒96.80; p = 0.029) were independent predictors of missed diagnoses.
CONCLUSION:
The availability of 24-hour ED specialist coverage and an on-site CT scanner improved the diagnosis of AAD in our study.
INTRODUCTION
Acute aortic dissection (AAD) is a rare condition occurring at a ratio of one AAD to every 12,200 emergency department (ED) patients.(1) AAD is a medical emergency that has a high mortality rate if not diagnosed and treated early. The rate of missed diagnosis of AAD in the ED was reported to be in the range of 16%‒38%.(2,3) As AAD has been reported to have varying atypical presentations, this condition may be underdiagnosed, leading to fatalities.(4) Unsurprisingly, AAD is among the conditions that lead to medical errors. A study done in Swedish EDs showed that one factor that contributed to diagnostic medical errors is lack of supervision over junior doctors.(5) Similarly, in the United States, an analysis of malpractice claims in the ED found that inadequate supervision was a contributing factor in 30% of cases.(6) The Royal College of Emergency Medicine, United Kingdom, and the Australian College for Emergency Medicine, Australia, identified the benefits of having a consultant presence in the ED and recommended a minimum of 16 hours’ coverage over seven days a week in their guidelines for the emergency medicine workforce.(7,8) In the most recent study,(9) process times for patients in the ED were reduced when 24-hour consultant coverage was available compared to coverage by middle-grade doctors.
According to the International Registry of AAD, computed tomography (CT) angiography is the most frequently used first diagnostic test (61%) in suspected aortic dissection, followed by echocardiography, thus reflecting the respective availability and accessibility of these modalities.(10) However, the logistics of transferring the patient to a CT room require the concerted efforts of the staff, particularly if the CT scanner is unavailable after hours or on-site. In a recent study of 1,696 patients who had acute thoracic and/or abdominopelvic CT performed in the EDs of two hospitals, the ED with an on-site CT scanner had faster throughput during all shifts; reductions in time to CT completion, interpretation by radiologists, and finalisation of disposition ranged from 15 to 19 minutes.(11) The amount of time reduced can be crucial in the outcomes of patients with AAD, for whom the mortality rate increases by 1%‒2% with each hour after symptom onset.(10)
The primary objective of this study was to determine if the availability of 24-hour ED specialist coverage and an on-site CT scanner reduced the rate of missed diagnoses of AAD. We hypothesised that there would be a decrease in the rate of missed diagnoses following the implementation of the changes. The secondary objective was to determine the predictors of missed diagnosis of AAD after implementation.
METHODS
This was a retrospective chart review conducted at the ED of a tertiary hospital, National University Hospital, Singapore, between January 1998 and December 2014. The ED received more than 120,000 attendances per year. The study was approved by the National Healthcare Group Domain Specific Review Board (ref no. 2014/01250) with a waiver of consent.
Records with a diagnosis of dissection of the aorta according to the International Classification of Diseases, Ninth Revision (code 441.0), were selected from the hospital’s discharge database and death register. Patients who were included in the study were: (a) diagnosed with AAD in the hospital discharge diagnosis and admitted from the ED; or (b) diagnosed with AAD as a cause of death and presented to the ED within five days from the date of death. The first criterion was used to identify cases that had been missed by the ED doctors and were subsequently diagnosed by the inpatient team. The second criterion was to identify patients who had attended the ED within five days prior to their death and had AAD as the final cause of death on postmortem. Patients who had been transferred from another hospital or admitted from the specialist outpatient clinics and those with known aortic dissection, planned elective admission for aortic dissection surgery, and aortic dissection that occurred as a complication of coronary artery bypass graft surgery were excluded.
Chart reviews were conducted using the hospital’s computerised patient records system: EDWeb (Emergency Department Web) and CPSS-2 (Computerised Patient Support System-2). Data collected from the chart reviews included patient demographics, past medical history, clinical presentation, clinical findings, electrocardiogram findings, imaging studies such as transthoracic echocardiography and CT, ED diagnosis and disposition, and in-hospital outcomes.
Before 2008, ED specialists did not consistently cover 24-hour shifts, leaving the remainder of the coverage to middle-grade doctors. Our centre has two postgraduate training programmes for emergency medicine. The first is a five-year training programme consisting of three years of basic specialist training followed by an intermediate exam and advanced specialist training in the next two years. The second is the residency programme whereby doctors spend five years in total as residents, with an intermediate exam at three years. In both programmes, trainees sit for an exit exam before qualifying as junior specialists. Middle-grade doctors are trainees who have completed the intermediate examination but have yet to complete their exit examinations. According to the departmental guidelines, the duty cardiologist or cardiothoracic surgeon needs to see the patient in the ED before CT can be requested. Upon assessing the patient and if indicated by the cardiologist or cardiothoracic surgeon, the duty radiologist is contacted via phone and the CT imaging is approved after a discussion of the case. The CT scanner was previously available at the radiology department for 24 hours a day. Patients needed to be transported with a nurse and a doctor from the ED to the CT suite, which is 130 m away from the ED and on a different floor. To receive the CT results, the ED physician had to call the duty radiologist who approved the scan earlier.
In November 2008, we introduced 24-hour board-certified ED physician (henceforth termed ‘specialists’) coverage in the ED. These specialists are available on-site at all times. In August 2009, we placed a dedicated CT scanner (Brilliance 64; Philips Medical Systems, Best, Netherlands) on-site in the ED, 17 m away from the resuscitation area. The specialists reviewed each case and made the decision to order CT imaging. A duty cardiologist or cardiothoracic surgeon may be consulted but their input is not mandatory.
A missed diagnosis of AAD was defined as the following: (a) during the pre-implementation period (before August 2009), not considering AAD as a differential diagnosis, not performing any imaging to diagnose AAD, or no cardiology or cardiothoracic surgery consultation being obtained while the patient was in the ED; and (b) during the post-implementation period, not considering AAD to be a differential diagnosis and not performing any imaging to diagnose AAD. The absence of documentation of AAD in chart reviews indicated that it was not considered as a differential diagnosis.
Data was analysed using PASW Statistics version 18.0 (SPSS Inc, Chicago, IL, USA). For the primary objective, univariate analysis was first performed to determine the rate of missed diagnoses between the two time periods. Student’s t-test was used for the continuous variable (i.e. age), while chi-square test or Fisher’s exact test was used for categorical variables. Subsequently, stepwise logistic regression was performed to adjust for confounders.(12) The confounders identified were variables that showed statistical significance (defined as p < 0.10) on univariate analyses or were deemed to affect the rate of missed diagnoses (i.e. age, syncope, paraplegia, pulse deficit, aortic regurgitation murmur, and pre- or post-implementation period). For the secondary objective, only patients from the post-implementation period were analysed. Similarly, a univariate analysis was performed followed by a stepwise logistic regression to determine factors that predicted missed diagnoses. Variables that showed statistical significance (defined as p < 0.10) on the univariate analyses (i.e. paraplegia, widened mediastinum on chest radiography and congestive cardiac failure [CCF]) were entered into the model. For both models, adjusted odds ratios (ORs) were expressed with a corresponding 95% confidence interval (CI).
RESULTS
A total of 145 patients were included in the analysis over the 17-year study period. The overall characteristics of the patients included in the pre- and post-implementation period are presented in
Table I
Patient characteristics in the pre- and post-implementation period.
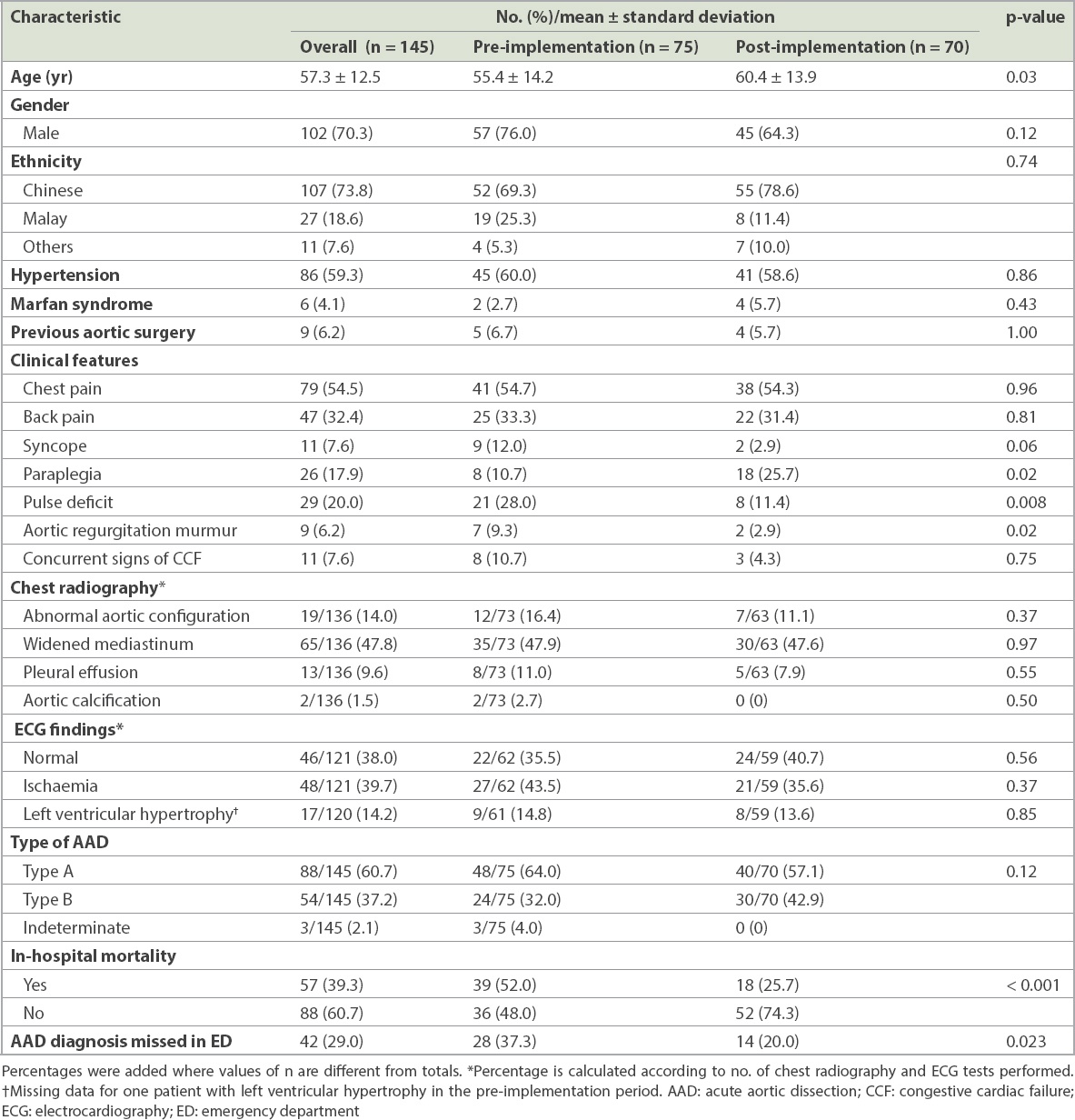
There was an increase in the number of AAD cases diagnosed (i.e. not missed) after the implementation of an on-site CT scanner and 24-hour specialist coverage in the ED (
Fig. 1
Chart shows the number of acute aortic dissection diagnoses by year. *2009 was the year when both 24-hour specialist coverage and an on-site computed tomography scanner became available.
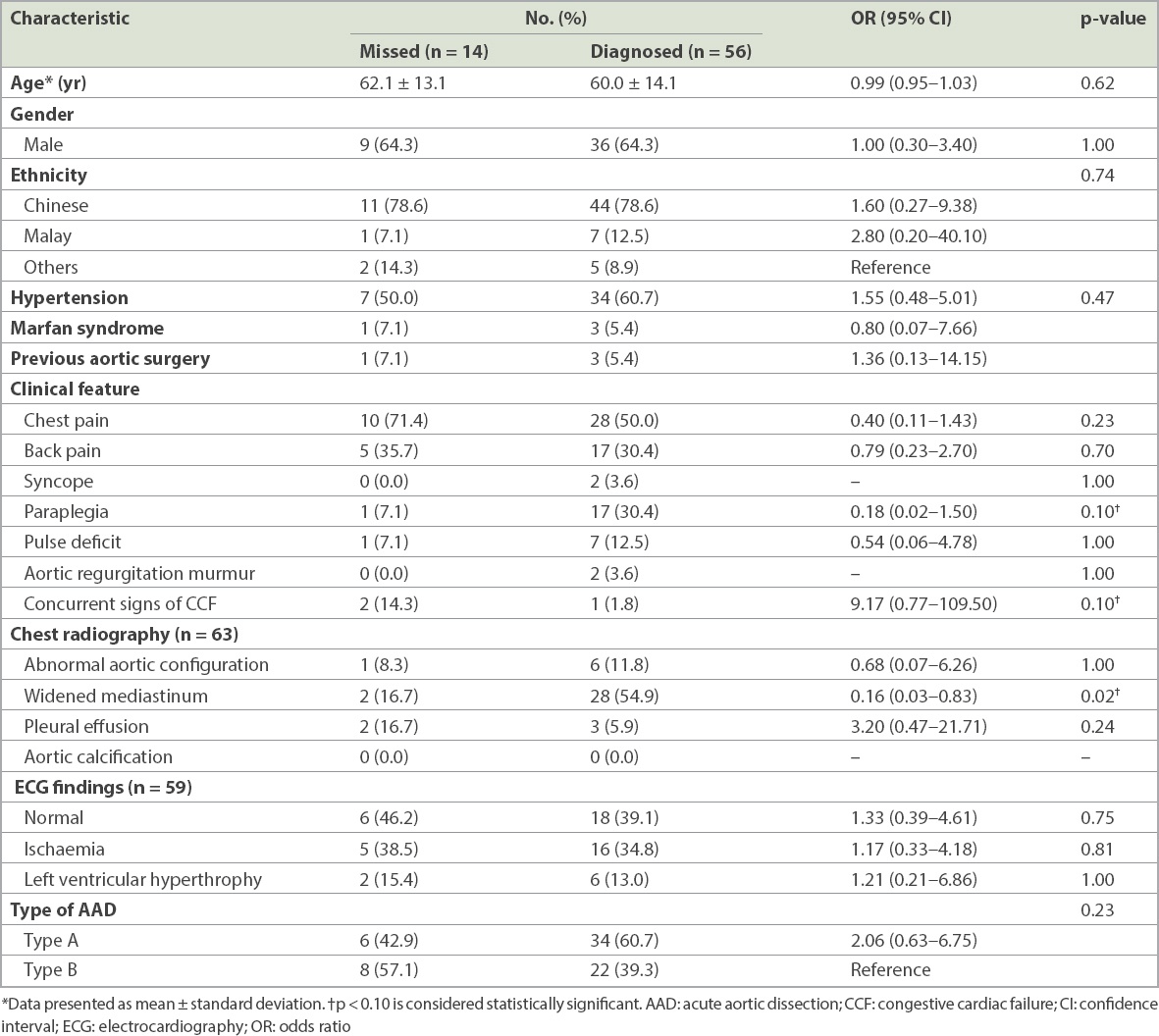
For the secondary objective, only the AAD cases in the post-implementation period (n = 70) were included for analysis (
Table II
Characteristics of patients in whom the diagnosis of AAD was made or missed in the post-implementation period.
DISCUSSION
Our study showed that the proportion of missed AAD diagnoses decreased after the implementation of 24-hour specialist coverage and an on-site CT scanner in the ED. There was a 17.3% increase in the proportion of diagnosed AAD despite a rise in the number of cases. The availability of the 24-hour ED specialist coverage has seemingly contributed to better diagnostic accuracy and more timely performance of relevant diagnostic tests.
Our results echoed those of previous studies, which also found 24-hour specialist coverage in the ED to be advantageous in several aspects such as the reduction of waiting time and admission rates.(9,13) Input from senior doctors likely adds accuracy to decisions that can improve patient safety. More than half of the malpractice claims in a United Kingdom study were attributed to missed ED diagnoses, the majority of which involved training doctors.(14) An increase in the number of consultants covering the ED can lower the rate of complaints, likely due to better patient management in terms of timely diagnosis, treatment and communication. However, at present, many EDs have yet to adopt 24-hour specialist coverage despite an open debate on this topic.(15-17) The results of our study can perhaps reinforce support for continuous specialist coverage.
Contrast-enhanced CT has evolved to be the most common preferred choice of investigation due to its high diagnostic accuracy of 92%‒96%.(18,19) It reliably confirms and excludes AAD and may help to elucidate alternative diagnoses of chest pain, including pulmonary embolism and obstructive coronary artery disease.(20) The availability of 24-hour consultant coverage and the on-site CT scanner helps to reduce the rate of misdiagnosis by allowing the ED specialist to make a decision on definitive imaging without the need for a consultation with a duty cardiologist or cardiothoracic surgeon. Apart from decreasing the time from symptom onset to definitive diagnosis and reducing the rate of misdiagnosis, making CT available in our ED without the need to transport a potentially ill patient to another radiological facility also minimises the potential risk of deterioration in the patient’s haemodynamic status during transfer to an offsite area.
Given the low incidence of AAD, it is important to re-evaluate and understand the potential contributors to a missed diagnosis. Before the implementation, we previously reported the absence of pulse deficit and widened mediastinum as independent predictors of misdiagnosis.(3) During the post-implementation period, only one of the two previously reported predictors, the absence of widened mediastinum on chest radiography, remained a significant predictor of misdiagnosis. The absence of pulse deficit no longer predicted missed diagnoses of AAD. We postulated that due to the availability and easy accessibility of the on-site CT scanner, clinicians were more likely to proceed with definitive imaging using CT aortography in patients with high clinical suspicion of AAD even if there was no pulse deficit. The utility of evaluations of widened mediastinum on chest radiography remains elusive, with varying sensitivity and specificity among different observers.(21)
However, our study found another clinical variable to be predictive of missed diagnoses: concurrent signs of CCF. CCF is a known potential complication of AAD, although its exact mechanism is not fully known. It has been postulated to be either secondary- to high-output cardiac failure or aortic regurgitation from direct dissection into the aortic valve leaflet.(22-25) Importantly, patients with concurrent CCF have been reported to present atypically, which may lead to a delay in the recognition and diagnosis of AAD.(26) This may account for our finding that CCF is an independent predictor for misdiagnosis. To prevent misdiagnoses, ED physicians should be aware of the possibility of concomitant pathologies. Clinical suspicion for AAD in patients presenting with CCF should remain high, particularly when the presentation of CCF is sudden and unexplained.
We acknowledge several limitations in our study. It is a retrospective study with inherent biases due to the study design. We cannot strongly conclude direct causation between the interventions and decrease in the misdiagnosis rate. However, given the low incidence of AAD, a prospective study may have been too time-consuming and inefficient. We were also limited by the amount of information reported in the chart reviews and may not have identified all variables. However, we included variables that were shown to be important in our previous study and the literature.
We recognise that as the two changes implemented by our department may have occurred almost simultaneously, it may not be possible to discern how much each change contributed to the decreased rate of missed diagnoses. Nevertheless, the key finding was that both changes were associated with decreased missed diagnoses and may suggest some synergism. Our study also found that there were many more cases of AAD in the post-implementation period. It can be argued that diagnoses were more accurate in the post-implementation period due to a learning curve. Treating physicians (not necessarily emergency physicians) may have become more aware of AAD through better education and learning from previous misses, such as the presentation of missed cases in morbidity rounds, leading to more careful evaluation and investigations. Another possible explanation could be that the ageing population experienced an increase in the prevalence of chronic medical diseases, such as hypertension, that are risk factors for AAD, as we found that patients in the post-implementation group were older. Additionally, this study was conducted in a single institution, and hence the results may not be generalisable to other populations. There is also a possibility that we may have missed some cases of AAD that presented to the ED. However, this number is likely to be small, as the databases that we used were standard databases in our institution. In spite of the limitations, our study highlights the utility of CT availability and specialist consultation in the ED and provides the groundwork for further quality improvements in clinical care as well as research that is fully representative of the cohort of patients with AAD.
In conclusion, the availability of 24-hour specialist coverage and an on-site CT scanner in the ED improves the diagnosis of AAD. The absence of the classical finding of a widened mediastinum on chest radiography and the presence of concurrent CCF are significant predictors of missed diagnoses of AAD. It cannot be overemphasised that the diagnosis of AAD in the ED is prompted by clinical findings and requires a high index of suspicion even before confirmatory imaging becomes useful.
ACKNOWLEDGEMENT
We thank the Medical Publications Support Unit of the National University Health System, Singapore, for their assistance.


